"Ion-derived solids" are solid salts such as Cu(NO3)2, NaOH, and the so-called ionic solids such as NaCl.
Even before G.N.Lewis developed his theory of the shared electron pair bond, it was believed that bonding in many solid salts could be satisfactorily explained on the basis of simple electrostatic forces between the positive and negative ions which are assumed to be the basic molecular units of these compounds. Lewis himself continued to recognize this distinction, which has continued to be a part of the tradition of chemistry; the shared electron pair bond is known as the covalent bond, while the other type is the ionic or electrovalent bond.
The covalent bond is formed when two atoms are able to share electrons:
![]()
whereas the ionic bond is formed when the "sharing" is so unequal that an electron from atom A is completely lost to atom B, resulting in a pair of ions:
![]()
The two extremes of electron sharing represented by the covalent and ionic models appear to be generally consistent with the observed properties of molecular and ionic solids and liquids. But does this mean that there are really two kinds of chemical bonds, ionic and covalent?
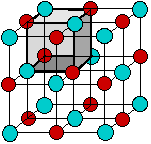 According to the ionic electrostatic model, solids such as NaCl consist of positive and negative ions arranged in a crystal lattice. Each ion is attracted to neighboring ions of opposite charge, and is repelled by ions of like charge; this combination of attractions and repulsions, acting in all directions, causes the ion to be tightly fixed in its own location in the crystal lattice.
According to the ionic electrostatic model, solids such as NaCl consist of positive and negative ions arranged in a crystal lattice. Each ion is attracted to neighboring ions of opposite charge, and is repelled by ions of like charge; this combination of attractions and repulsions, acting in all directions, causes the ion to be tightly fixed in its own location in the crystal lattice.
Since electrostatic forces are nondirectional, the structure of an ionic solid is determined purely by geometry: two kinds of ions, each with its own radius, will fall into whatever repeating pattern will achieve the lowest possible potential energy. Surprisingly, there are only a small number of possible structures.
When two elements form an ionic compound, is an electron really lost by one atom and transferred to the other one? In order to deal with this question, consider the data on the ionic solid LiF. The average radius of the neutral Li atom is about 2.52Å.
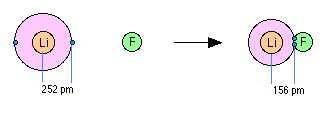
Now if this Li atom reacts with an atom of F to form LiF, what is the average distance between the Li nucleus and the electron it has "lost" to the fluorine atom? The answer is 1.56Å; the electron is now closer to the lithium nucleus than it was in neutral lithium! So the answer to the above question is both yes and no: yes, the electron that was now in the 2s orbital of Li is now within the grasp of a fluorine 2p orbital, but no, the electron is now even closer to the Li nucleus than before, so how can it be "lost"? The one thing that is inarguably true about LiF is that there are more electrons closer to positive nuclei than there are in the separated Li and F atoms. But this is just the condition that gives rise to all forms of chemical bonding:
Chemical bonds form when electrons can be simultaneously
near two or more nuclei.
It is obvious that the electron-pair bond brings about this situation, and this is the reason for the stability of the covalent bond. What is not so obvious (until you look at the numbers such as were quoted for LiF above) is that the "ionic" bond results in the same condition; even in the most highly ionic compounds, both electrons are close to both nuclei, and the resulting mutual attractions bind the nuclei together. This being the case, is there really any fundamental difference between the ionic and covalent bond?
The answer, according to modern chemical thinking is probably "no"; in fact, there is some question as to whether it is realistic to consider that these solids consist of "ions" in the usual sense. The preferred picture that seems to be emerging is one in which the electron orbitals of adjacent atom pairs are simply skewed so as to place more electron density around the "negative" element than around the "positive" one.
This being said, it must be reiterated that the ionic model of bonding is a useful one for many purposes, and there is nothing wrong with using the term "ionic bond" to describe the interactions between the atoms in "ionic solids" such as LiF and NaCl.
If there is no such thing as a "completely ionic" bond, can we have one that is completely covalent? The answer is yes, if the two nuclei have equal electron attracting powers. This situation is guaranteed to be the case with homonuclear diatomic molecules— molecules consisting of two identical atoms. Thus in Cl2, O2, and H2, electron sharing between the two identical atoms must be exactly even; in such molecules, the center of positive charge corresponds exactly to the center of negative charge: halfway between the two nuclei.
This term was introduced earlier in the course to denote the relative electron-attracting power of an atom. The electronegativity is not the same as the electron affinity; the latter measures the amount of energy released when an electron from an external source "falls into" a vacancy within the outermost orbital of the atom to yield an isolated negative ion.
The products of bond formation, in contrast, are not ions and they are not isolated; the two nuclei are now drawn closely together by attraction to the region of high electron density between them. Any shift of electron density toward one atom takes place at the energetic expense of stealing it from the other atom.
It is important to understand that electronegativity is not a measurable property of an atom in the sense that ionization energies and electron affinity are; electronegativity is a property that an atom displays when it is bonded to another. Any measurement one does make must necessarily depend on the properties of both of the atoms.
By convention, electronegativities are measured on a scale on which the highest value, 4.0, is arbitrarily assigned to fluorine. A number of electronegativity scales have been proposed, each based on slightly different criteria. The most well known of these is due to Linus Pauling, and is based on a study of bond energies in a variety of compounds.
The periodic trends in electronegativity are about what one would expect; the higher the nuclear charge and the smaller the atom, the more strongly attractive will it be to an outer-shell electron of an atom within binding distance. The division between the metallic and nonmetallic elements is largely that between those that have Pauling electronegativies greater than about 1.7, and those that have smaller electronegativities.
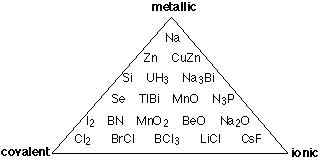
The greater the electronegativity difference between two elements A and B, the more polar will be their molecule AB. It is important to point out, however, that the pairs having the greatest electronegativity differences, the alkali halides, are solids order ordinary conditions and exist as molecules only in the rarefied conditions of the gas phase. Even these ionic solids possess a certain amount of covalent character, so, as discussed above, there is no such thing as a "purely ionic" bond. It has become more common to place binary compounds on a scale something like that shown here, in which the degree of shading is a rough indication of the number of compounds at any point on the covalent-ionic scale.
![]()
The covalent-ionic continuum described above is certainly an improvement over the old covalent -versus - ionic dichotomy that existed only in the textbook and classroom, but it is still only a one-dimensional view of a multidimensional world, and thus a view that hides more than it reveals.
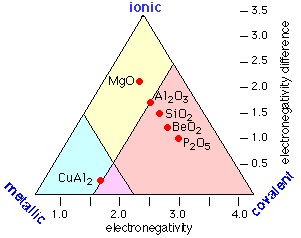
The main thing missing is any allowance for the type of bonding that occurs between more pairs of elements than any other: metallic bonding. Intermetallic compounds are rarely even mentioned in introductory courses, but since most of the elements are metals, there are a lot of them, and many play an important role in metallurgy. In metallic bonding, the valence electrons lose their association with individual atoms; they form what amounts to a mobile "electron fluid" that fills the space between the crystal lattice positions occupied by the atoms, (now essentially positive ions.) The more readily this electron delocalization occurs, the more "metallic" the element.
Thus instead of the one-dimension chart shown above, we can construct a triangular diagram whose corners represent the three extremes of "pure" covalent, ionic, and metallic bonding.
We can take this a step farther by taking into account collection of weaker binding effects known generally as van der Waals forces. Contrary to what is often implied in introductory textbooks, these are the major binding forces in most of the common salts that are not alkali halides; these include NaOH, CaCl2, MgSO4. They are also significant in solids such as CuCl2 and solid SO3 in which infinite covalently-bound chains are held together by ion-induced dipole and similar forces.
The only way to represent this four-dimensional bonding-type space in two dimensions is to draw a projection of a tetrahedron, each of its four corners representing the "pure" case of one type of bonding.
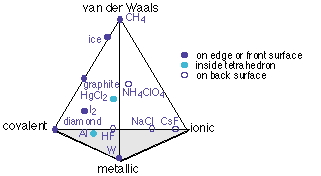 Note that some of the entries on this diagram (ice, CH4, and the two parts of NH4ClO4) are covalently bound units, and their placement refers to the binding between these units. Thus the H2O molecules in ice are held together mainly by hydrogen bonding, which is a van der Waals force, with only a small covalent contribution.
Note that some of the entries on this diagram (ice, CH4, and the two parts of NH4ClO4) are covalently bound units, and their placement refers to the binding between these units. Thus the H2O molecules in ice are held together mainly by hydrogen bonding, which is a van der Waals force, with only a small covalent contribution.
Note: the triangular and tetrahedral diagrams above were adapted from those in the excellent article by William B. Jensen, "Logic, history and the chemistry textbook", Part II, J. Chemical Education 1998: 817-828.
For a thorough discussion of chemical bonding,
see the Chem1 Chemical Bonding Reference Text