Atoms, Elements, and the Nucleus
Basic atomics - Getting Started
The idea of the atom — at one time a theory, but now directly observable — is the basic concept that unites all aspects of Chemistry, so this is where we begin. This lesson introduces you to these building-blocks of matter, and explains how they are characterized.


Atoms and elements: what's the difference?
The parallel concepts of the element and the atom constitute the very foundations of chemical science.

Sulfur the element

Sulfur the atom
An element is an actual physical substance that cannot be broken down into a simpler form, and is capable of an independent existence as observable matter. As such, the concept of the element is a macroscopic one that relates to the world that we can observe with our senses.
The atom is the microscopic realization of this concept; that is, it is the actual physical particle that is unique to each chemical element. Their very small size has long prevented atoms from being observable by direct means, so their existence was not universally accepted until the late 19th Century. The fact that we still hear mention of the "atomic theory of matter" should not imply that there is now any doubt about the existence of atoms; f ew theories in the history of science have been as thoroughly validated and are as well understood.
Although the word atom usually refers to a specific kind of particle (an "atom of magnesium", for example), our everyday use of "element" tends to be more general, referring not only to a substance composed of a particular type of atom ("bromine is one of the few elements that are liquids at room temperature"), but also to atoms in a collective sense ("magnesium is one of the elements having two electrons in its outer shell").
Atoms are not new!
 The underlying concept of atoms as the basic building blocks of matter has been around for a long time.
The underlying concept of atoms as the basic building blocks of matter has been around for a long time.
As early as 600 BCE, the Gujarati (Indian) philosopher Acharya Kanad wrote that "Every object of creation is made of atoms which in turn connect with each other to form molecules".
A couple of centuries later in 460 BCE, the Greek philosopher Democritus reasoned that if you keep breaking a piece of matter into smaller and smaller fragments, there will be some point at which the pieces cannot be made any smaller. He called these "basic matter particles"— in other words, atoms. But this was just philosophy; it would not become science until 1800 when John Dalton showed how the atomic concept followed naturally from the results of quantitative experiments based on weight measurements.
The element is the fundamental unit of chemical identity.
The philosophers' elements
The four elements of Western alchemy

The figure shows how the four elements were imagined to combine in various pairs to produce the "qualities" of hot, cold, wetness and dryness.
The concept of the element is an ancient one which developed in many different civilizations in an attempt to rationalize the variety of the world and to understand the nature of change, such as that which occurs when a piece of wood rots, or is burnt to produce charcoal or ash. Most well known to us are the four elements "earth, air, fire and water" that were popularized by Greek philosophers (principally Empedocoles and Aristotle) in the period 500-400 BCE.
To these, Vedic (Hindu) philosophers of India added space, while the ancient Chinese concept of Wu Xing regarded earth, metal, wood, fire and water as fundamental. These basic elements were not generally considered to exist as the actual materials we know as "earth", "water", etc., but rather represented the "principles" or essences that these elements contributed to the various kinds of matter we encounter in the world.
The chemists' elements

Antoine Lavoisier (1743-1794)
Eventually, practical experience (largely connected with the extraction of metals from ores) and the beginnings of scientific experimentation in the 18th Century led to our modern concept of the chemical element.
"Simplest", in the context of experimentation at the time, was defined in terms of weight; cinnabar (mercuric sulfide) can be broken down into two substances, mercury and sulfur, which themselves cannot be reduced to any lighter forms.

Although old Antoine got many of these right, he did manage to include a few things that don't quite fit into our modern idea of what constitutes a chemical element. There are two such mistakes in the top section of the table that you should be able to identify even if your French is less than tip-top— can you find them?
Lav's other mis-assignment of the elements in the bottom section was not really his fault. Chalk, magnesia, barytes, alumina and silica are highly stable oxygen-containing compounds; the high temperatures required to break them down could not be achieved in Lavoisier's time. (Magnesia, after all, is what fire brick is made of!) The proper classification of these substances was delayed until further experimentation revealed their true nature.
Ten of the chemical elements have been known since ancient times. Five more were discovered through the 17th Century.
Some frequently-asked questions about elements
How many elements are there?
Ninety-two elements have been found in nature. Around 25 more have been made artificially, but all of these decay into lighter elements, with some of them disappearing in minutes or even seconds.
Where do the elements come from?
The processes by which elements (or more properly, their nuclei) are formed is known as nucleosynthesis. The very first nuclei of the lightest elements (hydrogen and helium) formed about 20 minutes after the "big bang" 13.8 billion years ago.
The next 23 elements (up through iron) are continuouly being formed mostly by nuclear fusion processes within stars, in which lighter nuclei combine into successively heavier elements. Elements heavier than iron cannot be formed in this way, and are produced only during the catastrophic collapse of massive stars (supernovae explosions).
How do the elements vary in abundance?
Quite markedly, and very differently in different bodies in the cosmos. Most of the atoms in the universe still consist of hydrogen, with helium being a distant second. On Earth, oxygen, silicon, and aluminum are most abundant. These profiles serve as useful guides for constructing models for the formation of the earth and other planetary bodies.

Elemental abundances in the lithosphere (Earth's crust) and in the universe →
Note that the vertical axis is logarithmic, which has the effect of greatly reducing the visual impression of the differences between the various elements.
How did the elements get their names?
This is too big a subject to cover here in detail, especially since most elements have different names in different languages. Here are some useful links:
- ← By far the most interesting resource is the Elementymology Multidictionary which covers the history, discovery and naming of each element and a list of the names of each element in 97 languages.
- Wikipedia has a comprehensive list of English element name etymologies and also a Timeline of chemical element discoveries.
- The naming of some of the more recently-discovered (artificially-created) elements has been a matter of some controversy.
- An interesting description of how elements are named in Asian languages.
How did the element symbols originate?
How are the elements organized?
Two general organizing principles developed in the 19th Century: one was based on the increasing relative weights (atomic weights) of the elements, yielding a list that begins this way:
H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca...
The other principle took note of the similarities of the properties of the elements, organizing them into groups with similar properties (Döbereiner's "triads", 1829). It was later noted that groups of elements (Chancourtois' "telluric helix", Newlands "octaves", 1864 and Meyer, 1869) having changing properties tended to repeat themselves within the atomic weight sequence, giving rise to the idea of "periodic" sequences of properties. These concepts were finally integrated into the periodic table published by Mendeleev in 1869, which evolved into the various forms of the periodic table in use today.

"un-cut-able"
Throughout most of history the idea that matter is composed of minute particles had languished as a philosophical abstraction known as atomism, and no clear relation between these "atoms" and the chemical "elements" had been established. This began to change in the early 1800's when the development of balances that permitted reasonably precise measurements of the weight changes associated with chemical reactions ushered in a new and fruitful era of experimental chemistry. This resulted in the recognition of several laws of chemical change that laid the groundwork for the atomic theory of matter: conservation of mass, definite proportions, and multiple proportions.
Laws of chemical change
Recall that a "law", in the context of science, is just a relationship, discovered through experimentation, that is sufficiently well established to be regarded as beyond question for most practical purposes. Because it is the nature of scientists to question the "unquestionable", it occasionally happens that exceptions do arise, in which case the law must undergo appropriate modification.
Conservation of mass-energy in chemistry
Mass conservation is usually considered the most fundamental of law of nature. It is also a good example of a law that had to be modified; it was known simply as Conservation of Mass until Einstein showed that energy and mass are interchangeable. However, the older term is perfectly acceptable within the field of ordinary chemistry in which energy changes are too small to have a measurable effect on mass relations.
Within the context of chemistry, conservation of mass can be thought of as "conservation of atoms".
Chemical change just shuffles them around into new arrangements.
Mass conservation had special significance in understanding chemical changes involving gases, which were for some time not always regarded as real matter at all. (Owing to their very small densities, carrying out actual weight measurements on gases is quite difficult to do, and was far beyond the capabilities of the early experimenters.) Thus when magnesium metal is burned in air, the weight of the solid product always exceeds that of the original metal, implying that the process is one in which the metal combines with what might have been thought to be a "weightless" component of the air, which we now know to be oxygen.
![]()
More importantly, as we will see later, this experimental result tells us something very important about the mass of the oxygen atom relative to that of the magnesium atom.
Law of definite proportions
This law is also known as the law of constant composition. It states that the proportion by weight of the element present in any pure substance is always the same. This enables us to generalize the relationship we illustrated above.
The laws of definite and of multiple proportions, along with conservation of mass, are known collectively as the laws of chemical composition.
Law of multiple proportions
Many combinations of elements can react to form more than one compound. In such cases, this law states that the weights of one element that combine with a fixed weight of another of these elements are integer multiples of one another. It's easy to say this, but please take the time to make sure that you understand how it works!
Consider, for example, the five compounds of nitrogen and oxygen depicted in the chart shown here.

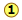 shows the ratio of the relative weights of the two elements in each compound. These ratios were calculated by simply taking the molar mass of each element, and multiplying by the number of atoms of that element per mole of the compound. Thus for NO2, we have (1 × 14) : (2 × 16) = 14:32. (These numbers were not known in the early days of Chemistry because atomic weights (i.e., molar masses) of most elements were not reliably known.)
shows the ratio of the relative weights of the two elements in each compound. These ratios were calculated by simply taking the molar mass of each element, and multiplying by the number of atoms of that element per mole of the compound. Thus for NO2, we have (1 × 14) : (2 × 16) = 14:32. (These numbers were not known in the early days of Chemistry because atomic weights (i.e., molar masses) of most elements were not reliably known.) are just the mass ratios of O:N, found by dividing the corresponding ratios in line 1. But someone who depends solely on experiment would work these out by finding the mass of O that combines with unit mass (1 g) of nitrogen.
are just the mass ratios of O:N, found by dividing the corresponding ratios in line 1. But someone who depends solely on experiment would work these out by finding the mass of O that combines with unit mass (1 g) of nitrogen. is obtained by dividing the figures the previous line by the smallest O:N ratio in the line above, which is the one for N2O. Note that just as the law of multiple proportions says, the weight of oxygen that combines with unit weight of nitrogen work out to small integers.
is obtained by dividing the figures the previous line by the smallest O:N ratio in the line above, which is the one for N2O. Note that just as the law of multiple proportions says, the weight of oxygen that combines with unit weight of nitrogen work out to small integers.
How Dalton's interpretation of the laws of chemical change established the atomic theory
The idea that matter is composed of tiny "atoms" of some kind had been around for at least 2000 years. Dalton's accomplishment was to identify atoms with actual chemical elements.

If Nobel prizes had existed in the early 1800's, the English schoolteacher/meteorologist/chemist John Dalton (1766-1844) would certainly have won one for showing how the experimental information available at that time, as embodied in the laws of chemical change that we have just described, are fully consistent with the hypothesis that atoms are the smallest units of chemical identity.
These points of Dalton's atomic theory provided satisfactory explanations of all the laws of chemical change noted above:
Explanation of the law of conservation of mass
This is really a consequence of "conservation of atoms" which are presumed to be indestructible by chemical means. In chemical reactions, the atoms are simply rearranged, but never destroyed.
Explanation of the law of constant composition
If compounds are made up of definite numbers of atoms, each of which has its own characteristic mass, then the relative mass of each element in a compound must always be the same. Thus the elements must always be present in a pure sample of a compound in the same proportions by mass.
Explanation of the law of multiple proportions
A given set of elements can usually form two or more compounds in which the numbers of atoms of some of the elements are different. Because these numbers must be integers (you can't have "half" an atom!), the mass of one element combined with a fixed mass of any other elements in any two such compounds can differ only by integer numbers. Thus, for the series of nitrogen-hydrogen compounds cited in the Problem Example above, we have the following relations:
| Compound | Formula | weight ratio H:N | ratio to 0.0714 |
|---|---|---|---|
| urea | CO(NH2)2 | 0.1428 | 2 |
| ammonia | NH3 | 0.0714 | 1 |
| ammonium chloride | NH4Cl | 0.2857 | 4 |
"Seeing" is believing: the atomic force microscope
Although Dalton's atomic theory was immediately found to be a useful tool for organizing chemical knowledge, it was some time before it became accepted as a true representation of the world. Thus, as late as 1887, one commentator observed that
"Atoms are round bits of wood invented by Mr. Dalton."
These wooden balls have evolved into computer-generated images derived from the atomic force microscope (AFM), an exquisitely sensitive electromechanical device in which the distance between the tip of a submicroscopic wire probe and the surface directly below it is recorded as the probe moves along a surface to which atoms are adsorbed.

Atoms of cobalt on a copper surface.

These xenon atoms on a nickel surface make up the world's smallest corporate logo. "Pictures" such as these are created by selectively removing individual atoms or molecules from the surface.
Dalton's atomic theory immediately led to the realization that although atoms are far too small to be studied directly, their relative masses can be estimated by observing the weights of elements that combine to form similar compounds. These weights are sometimes referred to as combining weights.
There is one difficulty, however: we need to know the formulas of the compounds we are considering in order to make valid comparisons. For example, we can find the relative masses of two atoms X and Y that combine with oxygen only if we assume that the values of n in the two formulas XOn and YOn are the same. But the very relative masses we are trying to find must be known in order to determine these formulas.
The way to work around this was to focus on binary (two-element) compounds that were assumed to have mostly simple atom ratios such as 1:1, 1:2, etc., and to hope that enough 1:1 compounds would be found to provide a starting point for comparing the various pairs of combining weights. Compounds of oxygen, known as oxides, played an especially important role here, partly because almost all of the chemical elements form compounds with oxygen, and most of them do have very simple formulas.
Discovering the formula of water
Of these oxygen compounds, the one with hydrogen— ordinary water— had been extensively studied. The first proof that water is composed of hydrogen and oxygen was the discovery, in 1800, that an electric current could decompose water into these elements. Notice the 2:1 volumes of the two gases displacing the water at the tops of the tubes. Although the significance of this volume ratio was not recognized at the time, it would later prove the formula we now know for water.
Earlier experiments had given the composition of water as 87.4 percent oxygen and 12.6 percent hydrogen by weight. This means that if the formula of water is assumed to be HO (as it was at the time — incorrectly), then the mass ratio of the two kinds of atoms must be O:H = 87.4/12.6 = 6.9. Later work corrected this figure to 8, but the wrong assumption about the formula of water would remain to plague chemistry for almost fifty years until studies on gas volumes (Avogadro's law) proved that water is H2O.
Dalton's table of relative atomic masses
Dalton fully acknowledged the tentative nature of weight ratios based on assumed simple formulas such as HO for water, but was nevertheless able to compile in 1810 a list of the relative weights of the atoms of some of the elements he investigated by observing weight changes in chemical reactions.
| hydrogen | carbon | oxygen | phosphorus | <iron | zinc | copper | lead |
| 1 | 5 | 5.4 | 7 | 9 | 13 | 50 | 56 |
Because hydrogen is the lightest element, it was assigned a relative weight of unity.
Once the correct chemical formulas of more compounds became known, more precise combining-weight studies eventually led to the relative masses of the atoms we know today as the atomic weights, which we discuss farther on.
The precise physical nature of atoms finally emerged from a series of elegant experiments carried out between 1895 and 1915 The most notable of these achievements was Ernest Rutherford's famous 1911 alpha-ray scattering experiment, which established that

• Almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric charge, reside.
The extremely small mass of the electron (1/1840 the mass of the hydrogen nucleus) causes it to behave as a quantum particle, which means that its location at any moment cannot be specified; the best we can do is describe its behavior in terms of the probability of its manifesting itself at any point in space. It is common (but somewhat misleading) to describe the volume of space in which the electrons of an atom have a significant probability of being found as the electron cloud. The latter has no definite outer boundary, so neither does the atom. The radius of an atom must be defined arbitrarily, such as the boundary in which the electron can be found with 95% probability. Atomic radii are typically 30-300 pm.
The nucleus is composed of protons and neutrons
The nucleus is itself composed of two kinds of particles. Protons are the carriers of positive electric charge in the nucleus; the proton charge is exactly the same as the electron charge, but of opposite sign. This means that in any [electrically neutral] atom, the number of protons in the nucleus (often referred to as the nuclear charge) is balanced by the same number of electrons outside the nucleus.
Because the electrons of an atom are in contact with the outside world, it is possible for one or more electrons to be lost, or some new ones to be added. The resulting electrically-charged atom is called an ion.
The other nuclear particle is the neutron. As its name implies, this particle carries no electrical charge. Its mass is almost the same as that of the proton. Most nuclei contain roughly equal numbers of neutrons and protons, so we can say that these two particles together account for almost all the mass of the atom.
The atomic number is the nuclear charge, and thus the number of electrons in the neutral atom
What single parameter uniquely characterizes the atom of a given element? It is not the atom's relative mass, as we will see in the section on isotopes below. It is, rather, the number of protons in the nucleus, which we call the atomic number and denote by the symbol Z. Each proton carries an electric charge of +1, so the atomic number also specifies the electric charge of the nucleus. In the neutral atom, the Z protons within the nucleus are balanced by Z electrons outside it.

Moseley searched for a measurable property of each element that increases linearly with atomic number. He found this in a class of X-rays emitted by an element when it is bombarded with electrons. The frequencies of these X-rays are unique to each element, and they increase uniformly in successive elements. Mosely found that the square roots of these frequencies give a straight line when plotted against Z; this enabled him to sort the elements in order of increasing atomic number.
Atomic numbers were first worked out in 1913 by Henry Moseley, a young member of Rutherford's research group in Manchester.
You can think of the atomic number as a kind of serial number of an element, commencing at 1 for hydrogen and increasing by one for each successive element. The chemical name of the element and its symbol are uniquely tied to the atomic number; thus the symbol "Sr" stands for strontium, whose atoms all have Z = 38.
Mass number
This is just the sum of the numbers of protons and neutrons in the nucleus. It is sometimes represented by the symbol A, so
A = Z + N
in which Z is the atomic number and N is the neutron number.
Nuclides and their symbols

Because it is not always easy to display a subscript directly beneath a superscript, it is not uncommon to use constructions such as 12Mg26 , which will often be our practice in this document when it is necessary to show both Z and A explicitly.
Isotopes are nuclides having the same atomic number

Approximately 290 isotopes occur in nature.
The two heavy isotopes of hydrogen are especially important— so much so that they have names and symbols of their own:

Deuterium accounts for only about 15 out of every one million atoms of hydrogen. Tritium, which is radioactive, is even less abundant. All the tritium on the earth is a by-product of the decay of other radioactive elements.
For historical reasons, the term atomic weight has a special meaning in Chemistry; it does not refer to the actual "weight" of an atom, which would be expressed in grams or kg.
Atomic weights, sometimes called relative weights, are more properly known as relative atomic masses, and being ratios, are dimensionless.
Please note that although the terms mass and weight have different meanings, the differences between their values are so small as to be insignificant for most practical purposes, so the terms atomic weight and atomic mass can be used interchangeably.
Atoms are of course far too small to be weighed directly; weight measurements can only be made on the massive (but unknown) numbers of atoms that are observed in chemical reactions. The early combining-weight experiments of Dalton and others established that hydrogen is the lightest of the atoms, but the crude nature of the measurements and uncertainties about the formulas of many compounds made it difficult to develop a reliable scale of the relative weights of atoms. Even the most exacting weight measurements we can make today are subject to experimental uncertainties that limit the precision to four significant figures at best.
Atomic weights are average relative masses
In the earlier discussion of relative weights of atoms, we explained how Dalton assigned a relative weight of unity to hydrogen, the lightest element, and used combining weights to estimate the relative weights of the others he studied. Later on, when it was recognized that more elements form simple compounds with oxygen, this element was used to define the atomic weight scale. Selecting O = 16 made it possible to retain values very close to those already assigned on the H=1 scale.
Finally, in 1961, carbon became the defining element of the atomic weight scale. But because, by this time, the existence of isotopes was known, it was decided to base the scale on one particular isotope of carbon, C-12, whose relative mass is defined as exactly 12.000. Because almost 99% of all carbon atoms on the earth consist of 6C12, atomic weights of elements on the current scale are almost identical to those on the older O=16 scale.
Most elements possess more than one stable isotope in proportions that are unique to each particular element. For this reason, atomic weights are really weighted averages of the relative masses of each that are found on earth.
Atomic weights are the ratios of the average mass of the atoms of an element to the mass of an identical number
of 6C12 atoms.
You can visualize the atomic weight scale as a long line of numbers that runs from 1 to around 280. The beginning of the scale looks like this:
For many elements, one particular isotope so dominates the natural mixture that the others have little effect on the average mass. For example, 99.99 percent of hydrogen atoms consist of 1H1, whereas 1H2, the other stable isotope, amounts to only 0.01 percent. Similarly, oxygen is dominated by
8O16 (over 99.7 percent) to the near exclusion of its two other isotopes.

You will notice that the precisions of these atomic weights, as indicated by the number of significant figures, vary considerably.
- Atomic weights of the 26 elements having a single stable isotope (monoisotopic elements) are the most precisely known. Two of these, boron and fluorine, appear in the above table.
- Owing to geochemical isotopic fractionation (discussed farther on), there is always some uncertainty in averaging the atomic weights of elements with two or more stable isotopes.
- Industrial processes associated mainly with nuclear energy and weapons production require the isolation or concentration of particular isotopes. When the by-product elements or compounds from which these isotopes have been depleted eventually get distributed in the environment or sold into the commercial marketplace, their atomic weights can vary from "official" values. This has occurred, for example, with lithium, whose isotope Li-6 has been used to produce hydrogen bombs.
- Naturally-occurring radioactive elements (all elements heavier than
82Pb) all gradually decay into lighter elements, most of which are themselves subject to radioactive decay. These radioactive decay chains eventually terminate in a stable element, the most common of which is one of the three stable isotopes of lead. Subsequent geochemical processes can cause lead ore bodies from such sources to mix with "primeval" Pb (derived from the cosmic dust that formed the solar system), leading to a range of possible average atomic weights. For these reasons, lead, with a listed average atomic weight of 207.2, has the least-certain mass of any stable element.
Weighing atoms: mass spectrometry
A major breakthrough in Chemistry occurred in 1913 when J.J. Thompson directed a beam of ionized neon atoms through both a magnetic field and an electrostatic field. Using a photographic plate as a detector, he found that the beam split into two parts, and suggested that these showed the existence of two isotopes of neon, now known to be Ne-20 and Ne-22.
This, combined with the finding made a year earlier by Wilhelm Wien that the degree of deflection of a particle in these fields is proportional to the ratio of its electric charge to its mass, opened the way to characterizing these otherwise invisible particles.
Thompson’s student F.W. Aston improved the apparatus, developing the first functional mass spectrometer, and he went on to identify 220 of the 287 isotopes found in nature; this won him a Nobel prize in 1921. His work revealed that the mass numbers of all isotopes are nearly integers (that is, integer multiples of the mass number 1 of the protons and neutrons that make up the nucleus.
Neutral atoms, having no charge, cannot be accelerated along a path so as to form a beam, nor can they be deflected. They can, however, be made to acquire electric charges by directing an electron beam at them, and this was one of the major innovations by Aston that made mass spectrometry practical.
Mass spectrometry begins with the injection of a vaporized sample into an ionization chamber where an electrical discharge causes it to become ionized. An accelerating voltage propels the ions through an electrostatic field that allows only those ions having a fixed velocity (that is, a given charge) to pass between the poles of a magnet. The magnetic field deflects the ions by an amount proportional to the charge-to-mass ratios. The separated ion beams are detected and their relative strengths are analyzed by a computer that displays the resulting mass spectrum. In modern devices, a computer also controls the accelerating voltage and electromagnet current so as to being successive ion beams into focus on the detector.

 The mass spectrometer has become one of the most widely used laboratory instruments. Mass spectrometry is now mostly used to identify molecules. Ionization usually breaks a molecule up into fragments having different charge-to-mass ratios, each molecule resulting in a unique "fingerprint" of particles whose origin can be deduced by a jigsaw puzzle-like reconstruction. For many years, "mass-spec" had been limited to small molecules, but with the development of novel ways of creating ions from molecules, it has now become a major tool for analyzing materials and large biomolecules, including proteins.
The mass spectrometer has become one of the most widely used laboratory instruments. Mass spectrometry is now mostly used to identify molecules. Ionization usually breaks a molecule up into fragments having different charge-to-mass ratios, each molecule resulting in a unique "fingerprint" of particles whose origin can be deduced by a jigsaw puzzle-like reconstruction. For many years, "mass-spec" had been limited to small molecules, but with the development of novel ways of creating ions from molecules, it has now become a major tool for analyzing materials and large biomolecules, including proteins.
The mass spectrum of magnesium shows that it consists of three isotopes of masses 24 through 26. The height of each peak shows the abundance of each isotope.

Isotopic mixtures and abundances
Only 26 of the elements that occur on the Earth exist as a single isotope; these are said to be monoisotopic. The remaining elements consist of mixtures of between two and ten isotopes. The total number of natural isotopes is 339; of these, 254 are stable, while the remainder are radioactive, meaning that they decay into stable isotopes.
Recalling that a given isotope (also known as a nuclide) is composed of protons and neutrons, each having a mass number of unity, it should be apparent that the mass number of a given nuclide will be an integer, as seen in the mass spectrum of magnesium above.
It also follows that the relative atomic masses (“atomic weights”) of monoisotopic elements will be very close to integers, while those of other elements, being weighted averages, can have any value.
When there are only two significantly abundant isotopes, you can estimate the relative abundances from the mass numbers and the average atomic weight.
Isotope effects: when different isotopes exhibit different chemical behavior
The chemical behavior of an element is governed by the number and arrangement of its electrons in relation to its nuclear charge (atomic number). Because these quantities are identical for all isotopes of a given element, they are generally considered to exhibit identical chemical properties.
However, it turns out that the mass differences between different isotopes can give rise to very slight differences in their physical behavior that can, in turn affect their chemical behavior as well. These isotope effects are most evident in the lighter elements, in which small differences in neutron number lead to greater differences in atomic mass.
Thus no element is more subject to isotope effects than hydrogen: an atom of "heavy hydrogen" 1H2 (also known as deuterium and often given the symbol D) has twice the mass of an atom of 1H2. When this isotope is combined with oxygen, the resulting "heavy water" D2O exhibits noticeably different physical and chemical properties: it melts at 3.8° C and boils at 101.4° C. D2O apparently interferes with cell division in organisms; mammals given only heavy water typically die in about a week.
When two or more elements whose atoms contain multiple isotopes are present in a molecule, numerous isotopic modifications become possible.
For example, the two stable isotopes of hydrogen and of oxygen
(O16 and O18) give rise to combinations such as H2O18, HDO16, etc., all of which are readily identifiable in the infrared spectra of water vapor.
The amount of the rare isotopes of oxygen and hydrogen in water varies enough from place to place that it is now possible to determine the age and source of a particular water sample with some precision. These differences are reflected in the H and O isotopic profiles of organisms. Thus the isotopic analysis of human hair can be a useful tool for crime investigations and anthropology research. See also this Microbe Forensics page, and this general resource on water isotopes.
Isotopic fractionation
Isotope effects manifest themselves in both physical and chemical changes. In general,
- Chemical bonds involving lighter isotopes tend to be more readily broken, so reactions that depend on the rupture of such bonds will lead to products that are slightly enriched in the lighter isotopes.
- Phase changes such as vaporization similarly favor the lighter isotopes. Thus when water vapor condenses in clouds to form rain, the heavier water isotopes become slightly more concentrted in the liquid phase.
These two effects give rise to isotopic fractionation as chemical substances move through the environment — or on a much smaller scale, through the various metabolic processes that occur in organisms. Over time, this leads to changes in the isotopic signatures of elements in different realms of the world that can reveal information that would otherwise be hidden.
The extent of isotopic fractionation depends on the temperature. This fact has been put to practical use to estimate the global average temperature in past times by measuring the degree of enrichment of the heavier isotopes of oxygen in glacial ice cores and also in ancient sediments containing the shells of microorganisms.
Atomic weights, molecular weights and formula weights
Molecules are composed of atoms, so a molecular weight is just the sum of the atomic weights of the elements it contains.
Because some solids are not made up of discrete molecules (sodium chloride, NaCl, and silica, SiO2 are common examples), the term formula weight is often used in place of molecular weight. In general, the terms molecular weight and formula weight are interchangeable.
Here again is the beginning of the atomic weight scale that you saw above:

You should understand by now that atomic weights are relative weights, based on a scale defined by 6C12 = 12. But what is the absolute weight of an atom, expressed in grams or kilograms? In other words, what actual mass does each unit on the atomic weight scale represent?
The answer is 1.66053886 × 10–27 kg. This quantity (whose value you do not need to memorize) is known as the unified atomic mass unit, denoted by the abbreviation u. (Some older texts leave off the "unified" part, and call it the amu.
Why such a hard-to-remember number? Well, that's just how Nature sometimes does things. Fortunately, you don't need to memorize this value, because you can easily calculate its value from Avogadro's number, NA, which you are expected to know:
1 u = 1/NA gram = 1 ÷ (1000 NA) Kg
... but more about that in the later lesson on moles.
Masses of the subatomic particles
Atoms are composed of protons, neutrons, and electrons, whose properties are shown below:
| particle | mass, g | mass, u | charge | symbol |
|---|---|---|---|---|
| electron | 9.1093897 × 10–28 | 5.48579903 × 10–4 | 1– | –10e |
| proton | 1.6726231 × 10–24 | 1.007276470 | 1+ | 11H+, 11p |
| neutron | 1.6749286 × 10–24 | 1.008664904 | 0 | 01n |
Two very important points you should note from this table:
- The mass of the electron is negligible compared to that of the two nuclear particles;
- The proton and neutron have masses that are almost, but not exactly, identical.
Nuclear masses and mass defect
As we mentioned in one of the problem examples above, the mass of a nucleus is always slightly different from the masses of the nucleons (protons and neutrons) of which it is composed. The difference, known as the mass defect, is related to the energy associated with the formation of the nucleus through Einstein's famous formula e = mc2. This is the one instance in chemistry in which conservation of mass-energy, rather than of mass alone, must be taken into account. But there is no need for you to be concerned with this in this part of the course.


