Colloids and their uses
The world of the "in between"
Sand, salt, and chalk dust are made up of chunks of solid particles, each containing huge numbers of molecules. You can usually see the individual particles directly, although the smallest ones might require some magnification. At the opposite end of the size scale, we have individual molecules which dissolve in liquids to form homogeneous solutions.
There is, however, a vast but largely hidden world in between: particles so tiny that they cannot be resolved by an optical microscope, or molecules so large that they begin to constitute a phase of their own when they are suspended in a liquid.
This is the world of colloids which we will survey in this lesson. As you will see, we encounter colloids in the food we eat, the consumer products we buy... and we ourselves are built largely of colloidal materials.
The realm of the tiny
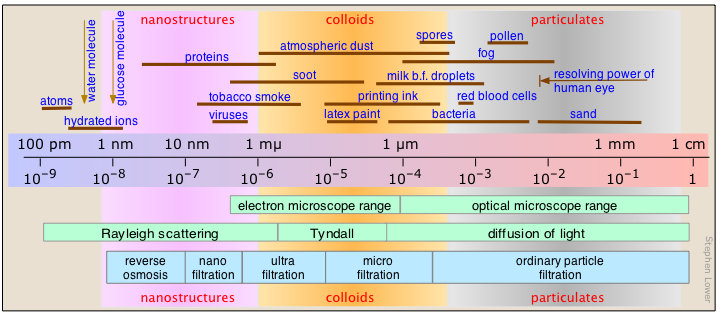
Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. In a sense, they bridge the microscopic and the macroscopic. As such, they possess some of the properties of both, which makes colloidal matter highly adaptable to specific uses and functions. Colloid science is central to biology, food science and numerous consumer products.
- Solutions are homogeneous mixtures whose component particles are individual molecules whose smallest dimension is generally less than 1 nm. Within this size range, thermal motions maintain homogeniety by overcoming the effects of gravitational attraction.
- Colloidal dispersions appear to be homogeneous, and the colloidal particles they contain are small enough (generally between 1-1000 nm) to exhibit Brownian motion, cannot be separated by filtration, and do not readily settle out. But these dispersions are inherently unstable and under certain circumstances, most colloidal dispersions can be "broken" and will "flocculate" or settle out.
- Suspensions are heterogeneous mixtures in which the suspended particles are sufficiently large (> 1000 nm in their smallest dimension) to settle out under the influence of gravity or centrifugal force. The particles that form suspensions are sometimes classified into various size ranges.
Colloidal particles need not fall within the indicated size range in all three dimensions; thus fibrous colliods such as many biopolymers may be very extended sizes along one direction.
The nature of colloidal particles
One phase or many?
To begin, you need to recall two important definitions:
- a phase is defined as a region of matter in which the composition and physical properties are uniform. Thus ice and liquid water, although two forms of the single substance H2O, constitute two separate phases within a heterogeneous mixture.
- A solution is a homogeneous mixture of two or more substances consisting of a single phase. (Think of sugar dissolved in water).
But imagine that you are able to shrink your view of a solution of sugar in water down to the sub-microscopic level at which individual molecules can be resolved: you would see some regions of space occupied by H2O molecules, others by sugar molecules, and likely still others in which sugar and H2O molecules are momentarily linked together by hydrogen bonding— not to mention the void spaces that are continually appearing and disappering between molecules as they are jumbled about by thermal motions.
As with so many simple definitions, the concept of homogeniety (and thus of a solution) breaks down as we move from the macro-scale into the molecular scale. And it is the region in between these two extremes that constitutes the realm of the colloid.
Smaller is bigger
What makes colloidal particles so special is not so much their sizes as it is the manner in which their surface areas increase as their sizes decrease. If we take a sample of matter and cut it up into smaller and smaller chunks, the total surface area will increase very rapidly.
Although mass is conserved, surface area is not; as a solid is sliced up into smaller bits, more surfaces are created. These new surfaces are smaller, but there are many more of them; the ratio of surface area to mass can become extremely large.
The total surface area increases as the inverse cube of the the face length, so as we make our slices still smaller, the total surface area grows rapidly.
In practical situations with real colloids, surface areas can reach hectares (or acres) per mole!
| number of slices per cube face | length of each face (cm) | surface area per face | number of cubes | total surface area |
|---|---|---|---|---|
| 0 | 1 | 1 cm2 | 1 | 6 cm2 |
| 10 | 0.1 | 0.01 cm2 | 1000 | 60 cm2 |
| 100 | 0.01 | 10–4 cm2 | 106 | 600 cm2 |
| 1000 | 10–3 | 10–6 cm2 | 109 | 0.6 m2 |
| n | 1/n | n–2 cm2 | n3 | 6n cm2 |
In the world of colloids, surface is king!
Why do we focus so much attention on surface area? The general answer is that surfaces (or more generally, interfaces between phases) possess physical and chemical properties of their own. In particular,
- Surfaces can exert van der Waals attractive forces on other molecules near them, and thus loosely bind other particles by adsorption
- Interfaces beteen different phases usually give rise to imbalances in electrical charge which can cause them to interact with nearby ions.
- The surfaces of many solids present "broken bonds" which are chemically active.
In normal "bulk" matter, these properties are mostly hidden from us owing to the small amount of surface area in relation to the quantity of matter. But as the particle size diminishes, surface phenomena begin to dominate their properties.
The small sizes of colloidal solids allows the properties of their surfaces to dominate their behavior.
Colloidal matter commonly exists in the form of colloidal-sized phases of solids, liquids, or gases that are uniformly dispersed in a separate medium (sometimes called the dispersions phase) which may itself be a solid, liquid, or gas. Colloids are often classified and given special names according to the particular kinds of phases involved.
| dispersed phase | medium | dispersion type | examples |
|---|---|---|---|
| gas | liquid | foam | whipped cream |
gas |
solid | solid foam | pumice1, aerogels2 |
| liquid | gas | liquid aerosol | fog, clouds |
| liquid | liquid | emulsion | milk3, mayonaisse, salad dressing |
| liquid | solid | gel | Jell-O, lubricating greases, opal4 |
| solid | gas | solid aerosol | smoke |
| solid | liquid | sol | paints, some inks, blood |
| solid | solid | solid sol | bone, colored glass, many alloys |
Large molecules can behave as colloids
Very large polymeric molecules such as proteins, starches and other biological polymers, as well as many natural polymers, exhibit colloidal behavior. There is no clear point at which a molecule becomes sufficiently large to behave as a colloidal particle.
Are colloids macroscopic or microscopic?
Colloidal dispersions behave very much like solutions in that they appear to be homogeneous on a macroscopic scale. They are often said to be microheterogeneous. The most important feature that distinguishes them from other particulate matter is that
Colloids dispersed in liquids or gases are sufficiently small that they do not settle out under the influence of gravity.
This, together with the their small sizes which allows them to pass through most filters, makes it difficult to separate colloidal matter from the phase in which it is dispersed.
Optical properties of colloidal dispersions
Colloidal dispersions are distinguished from true solutions by their light-scattering properties. The nature of this scattering depends on the ratio of the particle size in the medium to the wavelength of the light.
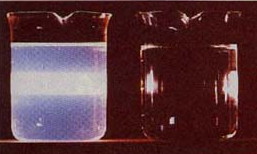 A collimated beam of light passing through a solution composed of ordinary molecules (r) tends retain its shape. When such a beam is directed through a colloidal dispersion, it spreads out (left container).→
A collimated beam of light passing through a solution composed of ordinary molecules (r) tends retain its shape. When such a beam is directed through a colloidal dispersion, it spreads out (left container).→
-
John Tyndall (1820-1893) was an Irish-born scientist whose interests spanned a remarkably wide scope, ranging from the nature of radiant heat to the flow of glaciers.

John Tyndall discovered this effect in 1869. Tyndall scattering (as it is commonly known) scatters all wavelengths equally. This is in contrast to Rayleigh scattering, which scatters shorter wavelengths more, bringing us blue skies and red sunsets.
Tyndall scattering can be seen even in dispersions that are transparent. As the density of particles (or the particle size) increases, the light scattering may become great enough to produce a "cloudy" effect, as in this image of a smoke-filled room → .
This is the reason that milk, fog, and clouds themselves appear to be white.
The individual water droplets in clouds (or the butterfat droplets in milk) are actually transparent, but the intense light scattering disperses the light in all directions, preventing us from seeing through them.
Visualizing colloids: the Ultramicroscope
Colloidal particles are, like molecules, too small to be visible though an ordinary optical microscope. However, if one looks in a direction perpendicular to the light beam, a colloidal particle will "appear" over a dark background as a tiny speck due to the Tyndall scattering. A microscope specially designed for this application is known as an ultramicroscope. Bear in mind that the ultramicroscope (invented in Austria in 1902) does not really allow us to "see" the particle; the scattered light merely indicates where it is at any given instant.
Brownian motion of colloidal particles
If you observe a single colloidal particle through the ultramicroscope, you will notice that it is continually jumping around in an irregular manner. These movements are known as Brownian motion. Scottish botanist Robert Brown discovered this effect in 1827 when observing pollen particles floating in water through a microscope. (Pollen particles are larger than colloids, but they are still small enough to exhibit some Brownian motion.)
It is worth noting that Albert Einstein's analysis of Brownian motion in 1901 constituted the first proof of the molecular theory of matter.
Brownian motion arises from collisions of the liquid molecules with the solid particle. For large particles, the millions of collisions from different directions cancel out, so they remain stationary. The smaller the particle, the smaller the number of surrounding molecules able to collide with it, and the more likely will random fluctuations occur in the number of collisions from different sides. Simple statistics predicts that every once in a while, the imbalance in collisions from different directions will become great enough to give the particle a real kick!
Electrical properties of colloids
In general, differences in electric potential exist between all phase boundaries.
If you have studied electrochemisty, you will know that two dissimilar metals in contact exhibit a "contact potential", and that similar potential differences exist between a metal and a solution in which it is immersed. But this principle extends well beyond ordinary electrochemisty; there are small potential differences even at the water-glass interface in a drinking glass, and the water-air interface above it.
Colloids are no exception to this rule; there is always a difference in electric potential between the colloid "phase" and that of the surrounding liquid. Even if the liquid consists of pure water, the polar H2O molecules at the colloid's surface are likely to be predominantly oriented with either their oxygen (negative) or hydrogen (positive) ends facing the interface, depending on the electrical properties of the colloid particle itself.
Interfacial electrical potential differences can have a variety of origins:
- Particles composed of ionic or ionizable substances usually have surface charges due to adsorption of an ion (usually an anion) from the solution, or to selective loss of one kinds of ion from the crystal surface. For example, Ag+ ions on the surface of a silver iodide crystal go into solution more readily than the Br- ions, leaving a negatively-charged surface.
- The charges of amphiprotic groups such as those on the surfaces of metal oxides and hydroxides will vary with the pH of the aqueous medium. Thus a particle of a metal oxide M–O will become positive in acidic solution due to formation of M–OH+, while that of a sparingly soluble hydroxide M–OH will become negative at low pH as it changes to M–O–. Colloidal-sized protein molecule can behave in a similar manner owing to the behavior of amphiprotic carboxylate-, amino- and sulfhydryl groups.
- Non-ionic particles or droplets such as oils or latex will tend to selectively adsorb positive or negative ions present in solution, thus "coating themselves" with electrical charge.
- In clays and other complex structures, isomorphous replacement of one ion by another having a different charge will leave a net electric charge on the particle. Thus particles of kaolinite clay become negatively charged due to replacement of some of the Si4+ ions by Al3+.
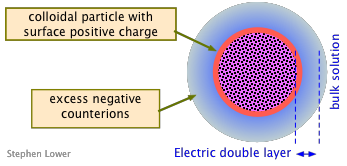 Charged colloidal particles will attract an exess of oppositely-charged counter-ions to their vicinity from the bulk solution, forming a localized "cloud" of compensating charge around each particle. The entire assembly is called an electric double layer.
Electric double layers of one kind or another exist at all phase boundaries, but those associated with colloids are especially important.
Charged colloidal particles will attract an exess of oppositely-charged counter-ions to their vicinity from the bulk solution, forming a localized "cloud" of compensating charge around each particle. The entire assembly is called an electric double layer.
Electric double layers of one kind or another exist at all phase boundaries, but those associated with colloids are especially important.
What keeps the colloidal particles suspended in the dispersion medium? How can we force the particles to settle out? These are very important practical matters:
- Colloidal products such as paints and many foods (e.g., milk) must remain in dispersed form if they are to be useful;
- Other dispersions, often those formed as by-products of operations such as mining, water treatment, paper-manufacture, or combusion are environmental nuisances. The only practical way of disposing of them is to separate the colloidal material from the much greater volume of the dispersion medium (most commonly water). Simple evaporation of the water is usually not a practical option; it is generally too slow, or too expensive if forced by heating.
A balance of forces
You will recall that weak attractive forces act between matter of all kinds. These are known generally as van der Waals and dispersion forces, and they only "take hold" at very close distances. Countering these is the universal repulsive force that acts at even shorter distances, but is far stronger; it is the basic reason why two atoms cannot occupy the same space.
For very small atomic- and molecular-sized particles, another thing that keeps them apart is thermal motion. Thus when two molecules in a gas collide, they do so with more than enough kinetic energy to overcome the weak attractive forces between them. As the temperature of the gas is reduced, so is the collisional energy; below its boiling point, the attractive forces dominate and the gas condenses into a liquid.
How electrical forces help keep colloids dispersed
When particles of colloidal dimension suspended in a liquid collide with each other, they do so with much smaller kinetic energies than is the case for gases, so in the absence of any compensating repulsion forces, we might expect van der Waals or dispersion attractions to win out. This would quickly result in the growth of aggregates sufficiently large to exceed colloidal size and to fall to the bottom of the container. This process is called coagulation.
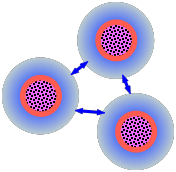 So how do stable dispersions such as sols manage to survive? In the preceding section, we saw that each particle with its double layer is more or less electrically neutral. However, when two particles approach each other, each one "sees" mainly the outer part [shown here in blue] of the double layer of the other. These will always have the same charge sign (which depends on the type of colloid and the nature of the medium), so there will be an electrostatic repulsive force that opposes the dispersion force attractions.
So how do stable dispersions such as sols manage to survive? In the preceding section, we saw that each particle with its double layer is more or less electrically neutral. However, when two particles approach each other, each one "sees" mainly the outer part [shown here in blue] of the double layer of the other. These will always have the same charge sign (which depends on the type of colloid and the nature of the medium), so there will be an electrostatic repulsive force that opposes the dispersion force attractions.
Electrostatic (coulumbic) forces have a strong advantage in this respect because they act over much greater distances do van der Waals forces.
But as we will see further on, electrostatic repulsion can lose its effectiveness if the ionic concentration of the medium is too great, or if the medium freezes. Under these conditions, there are other mechanisms that can stabilize colloidal dispersions.
How colloids interact with solvents
Colloids can be divided into two general classes according to how the particles interact with the dispersions medium (often referred to as the "solvent").
Lyophilic colloids
In one class of colloids, called lyophilic ("solvent loving") colloids, the particles contain chemical groups that interact strongly with the solvent, creating a sheath of solvent molecules that physically prevent the particles from coming together.
Ordinary gelatine is a common example of a lyophilic colloid. It is in fact hydrophilic, since it forms strong hydrogen bonds with water. When you mix Jell-O or tapioca powder to make a gelatine dessert, the material takes up water and forms a stable colloidal gel.
Lyophilic (hydrophilic) colloids are very common in biological systems and in foods.
Lyophobic colloids
Most of the colloids in manufactured products exhibit very little attraction to water: think of oil emulsions or glacially-produced rock dust in river water. These colloids are said to be lyophobic.
Lyophobic colloids are all inherently unstable; they will eventually coagulate. However, "eventually" can be a very long time (the settling time for some clay colloids in the ocean is 200-600 years!).
For systems in which coagulation proceeds too rapidly, the process can be slowed down by adding a stabilizer. Stabilizers can act by coating the particles with a protective layer such as a polymer as described immediately below, or by providing an ion that is selectively adsorbed by the particle, thereby surrounding it with a charged sheath that will repel similar particles it collides with.
Dispersions of these colloids are stabilized by electrostatic repulsion between the electric double layers surrounding the particles which we discussed in the preceding section.
How lyophobic colloids can masquerade as lyophilic
"Stabilization by stealth" has unwittingly been employed since ancient times through the use of natural gums to stabilize pigment particles in inks, paints, and pottery glazes. These gums are also widely used to stabilize foods and personal care products.
← A lyophobic colloid can be made to masquerade as lyophilic by coating it with something that itself possesses suitable lyophilic properties. This proceess is often referred to as "cloaking".
Steric stabilization
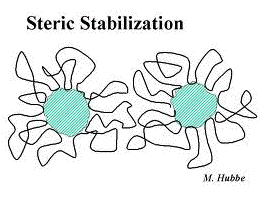
Alternatively, attaching a lyophobic material to a colloid of any type can surround the particles with a protective shield that physically prevents the particles from approaching close enough to join together. This method usually employs synthetic polymers and is often referred to as steric stabilization.
Synthetic polymers, which can be tailor-made for specific applications, are now widely employed for both purposes. The polymer can be attached to the central particle either by simple adsorption or by chemical bond formation.
Surfactants and micelle formation
Surfactants and detergents are basically the same thing. Surfactants that serve as cleaning agents are commonly called detergents (from L. detergere "to wipe away, cleanse").
Surfactants are molecules consisting of a hydrophylic "head" connected to a hydrophobic chain. Because such molecules can interact with both "oil" and water phases, they are often said to be amphiphilic. Typical of these is the well known cleaning detergent sodium dodecyl sulfonate ("sodium laurel sulfate") CH3(CH2)11OSO3– Na+.


 Amphiphiles possess the very important property of being able to span an oil-water interface. By doing so, they can stabilize emulsions of both the water-in-oil and oil-in-water types. Such molecules are essential components of the lipid bilayers that surround the cells and cellular organelles of living organisms.
Amphiphiles possess the very important property of being able to span an oil-water interface. By doing so, they can stabilize emulsions of both the water-in-oil and oil-in-water types. Such molecules are essential components of the lipid bilayers that surround the cells and cellular organelles of living organisms.

Emulsions are inherently unstable; left alone, they tend to separate into "oil" and "water" phases. Think of a simple salad dressing made by shaking vegetable oil and vineger. When a detergent-like molecule is employed to stabilize an emulsion, it is often referred to as an emulsifier. The resulting structure (left) is known as a micelle.
Emulsifiers are essential components of many foods. They are widely employed in pharmaceuticals, consumer goods such as lotions and other personal care products, paints and printing inks, and numerous industrial processes.
How detergents remove "dirt"
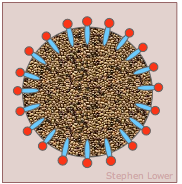
 The "dirt" we are trying to remove consists of oily or greasy materials whose hydrophobic nature makes them resistant to the action of pure water. If the water contains amphiphilic molecules such as soaps or cleaning detergents that can embed their hydrophobic ends in the particles, the latter will present a hydrophilic interface to the water and will thus become "solubilized".
The "dirt" we are trying to remove consists of oily or greasy materials whose hydrophobic nature makes them resistant to the action of pure water. If the water contains amphiphilic molecules such as soaps or cleaning detergents that can embed their hydrophobic ends in the particles, the latter will present a hydrophilic interface to the water and will thus become "solubilized".
Soaps and detergents can also disrupt the cell membranes of many types of bacteria, for which they serve as disinfectants. However, they are generally ineffective against viruses, which do not possess cell membranes.
Bile: your body's own detergent
Oils and fats are important components of our diets, but being insoluble in water, they are unable to mix intimately with the aqueous fluid in the digestive tract in which the digestive enzymes are dissolved. In order to enable the lipase enzymes (produced by the pancreas) to break down these lipids into their component fatty acids, our livers produce a mixture of sufactants known as bile. The great surface area of the micelles in the resulting emulsion enables efficient contact between the lipase enzymes and the lipid materials.
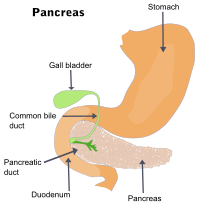
The liver of the average adult produces about 500 mL of bile per day. Most of this is stored in the gall bladder, where it is concentrated five-fold by removal of water. As partially-digested material exits the stomach, the gall bladder squeezes bile into the top of the small intestine (the duodenum).
 In addition to its action as a detergent (which also aids in the destruction of bacteria that may have survived the high acicity of the gastric fluid), the alkaline nature of the bile salts neutralizes the acidity of the stomach exudate.
In addition to its action as a detergent (which also aids in the destruction of bacteria that may have survived the high acicity of the gastric fluid), the alkaline nature of the bile salts neutralizes the acidity of the stomach exudate.
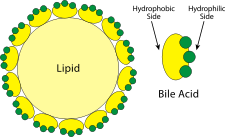
The bile itself consists of of salts of a variety of bile acids, all of which are derived from cholesterol. The cholesterol-like part of the structure is hydrophobic, while the charged end of the salt is hydrophilic. ↑ [WikiMedia]
Microemulsions

The microemulsion (right) is transparent, in contrast to the ordinary macroemulsion on the left.
Ordinary emulsions are inherently unstable; they do not form spontaneously, and once formed, the drop sizes are sufficiently large to scatter light, producing a milky appearance. As time passes, the average drop size tends to increase, eventually resulting in gravitational separation of the phases.
Microemulsions, in contrast, are thermodynamically stable and can form spontaneously. The drop radii are at the very low end of the colloidal scale, often 100 nm or smaller. This is too small to appreciably scatter visible light, so microemulsions appear visually to be homogenous systems.
Microemulsions require the presence of one or more surfactants which increase the flexibility and stability of the boundary regions. This allows them to vary form smaller micelles than surface tension forces would ordinarily allow; in some cases they can form sponge-like bicontinuous mixtures in which "oil" and "water" phases extend throughout the mixture, affording more contact area between the phases.
The uses of microemulsions are quite wide-ranging, with drug delivery, polymer synthesis, enzyme-assisted synthesis, coatings, and enhanced oil recovery being especially prominent.
A liquid phase dispersed in a solid medium is known as a gel, but this formal definition does not always convey the full sense of the nature of the "solid". The latter may start out as a powdery or granulated material such as natural gelatin or a hydrophilic polymer, but once the gel has formed, the "solid" part is less a "phase" than a cross-linked network that extends throughout the volume of the liquid, whose quantity largely defines the volume of the entire gel.
Hydrogels can contain up to 90% water by weight
Most of the gels we commonly encounter have water as the liquid phase, and thus are called hydrogels; ordinary gelatin deserts are well known examples.
The "solid" components of hydrogels are usually polymeric materials that have an abundance of hydrophilic groups such as hydroxyl (–OH) that readily hydrogen-bond to water and also to each other, creating an irregular, flexible, and greatly-extendable network. These polymers are sometimes synthesized for this purpose, but are more commonly formed by processing naturalmaterials, including natural polymers such as cellulose.
- Gelatine is a protein-like material made by breaking down the connective tissues of animal skins, organs, and bones. The many polar groups on the resulting protein fragments bind them together, along with water molecules, to form a gel.
- A number of so-called super-absorbant polymers derived from cellulose, polyvinyl alcohol and other materials can absorb huge quantities of water, and have found uses for products such as disposable diapers, environmental spill control, water retention media for plants, surgical pads and wound dressings, and protective inner coatings and water-blockers in fiber optics and electrical cables.
Gels are essential components of a huge variety of consumer products ranging from thickening agents in foods and personal care products to cushioning agents in running shoes.
Gels can be fragile!
You may have noticed that a newly-opened container of yoghurt or sour cream appears to be smooth and firm, but once some of the material has been spooned out, little puddles of liquid appear in the hollowed-out depressions.
As the spoon is plunged into the material, it pulls the nearby layers of the gel along with it, creating a shearing action that breaks it apart, releasing the liquid. Anyone who has attacked an egg yolk with a cook's wisk, written with a ball-point pen, or spread latex paint on a wall has made use of this phenomenon which is known as shear thinning.
Our bodies are mostly gels
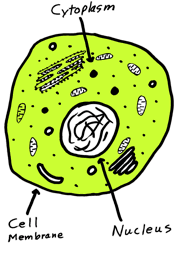
The interior (the cytoplasm) of each cell in the soft tissues of our bodies consists of a variety of inclusions (organelles) suspended in a gel-like liquid phase called the cytosol. Dissolved in the cytosol are a variety of ions and molecules varying from the small to the large; among the latter, proteins and carbohydrates make up the "solid" portion of the gel structure. [image] →
 Embedded within the cytosol is the filament-like cytoskeleton which controls the overall shape of the cell and holds the organelles in place.
Embedded within the cytosol is the filament-like cytoskeleton which controls the overall shape of the cell and holds the organelles in place.
← [image+] (In free-living cells such as the amoeba, changes in the cytoskeleton enable the organism to alter its shape and move around to engulf food particles.)
Be thankful for the gels in your body; without them, you would be little more than a bag of gunge-filled liquid, likely to end up as a puddle on the floor!
The individual cells are bound into tissues by the extracellular matrix (ECM) which — on a much larger scale, holds us together and confers an overall structure and shape to the body. The ECM is made of a variety of structural fibres (collagens, elastins) embedded in a gel-like matrix.
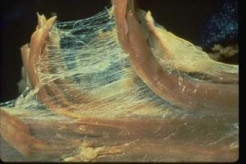
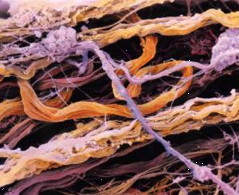 A good general description of the ECM can be found at the anatomytrains.com site, from which these two images were obtained. For much more detail, see this Wikipedia page.
A good general description of the ECM can be found at the anatomytrains.com site, from which these two images were obtained. For much more detail, see this Wikipedia page.
Cells, Gels and the Engines of Life: A New, Unifying Approach to Cell Function is the title of a fascinating, beautifully illustrated book by Gerald Pollack of the University of Washington. His central theme relates to the structuring effect of the water molecule on the dynamics of the cytoplasmic gel. As with most theories that challenge conventional scientific understanding, Pollack's ideas have attracted a lot of criticism from the scientific community (example), but as this review in Nature suggests, there is a lot here that is certainly worth exploring. This video of his 2009 UW Faculty Lecture is entertaining and informative.
Colloids as thickening agents
The usefulness of many industrial and consumer products is strongly dependent on their viscosity and flow properties. Toothpastes, lotions, lubricants, coatings are common examples. Most of the additives that confer desirable flow properties on these products are colloidal in nature; in many cases, they also provide stabilization and prevent phase separation. Since ancient times, various natural gums have been employed for such purposes, and many remain in use today.
More recently, manufactured materials whose properties can be tailored for specific applications have become widely available. Examples are colloidal microcrystalline cellulose, carboxymethyl cellulose, and fumed silica.
Fumed silica is a fine (5-50 nm), powdery form of SiO2 of exceptionally low bulk density (as little as 0.002 g cm–3); the total surface area of one Kg can be as great as 60 hectares (148 acres). It is made by spraying SiCl4(a liquid) into a flame. It is used as a filler, for viscosity and flow control, a gelling agent, and as an additive for strenghtening concrete.
Colloids in foods
 Most of the foods we eat are largely colloidal in nature. The function of food colloids generally has less to do with nutritional value than appearance, texture, and "mouth feel". The latter two terms relate to the flow properties of the material, such as spreadability and the ability to "melt" (transform from gel to liquid emulsion) on contact with the warmth of the mouth.
Most of the foods we eat are largely colloidal in nature. The function of food colloids generally has less to do with nutritional value than appearance, texture, and "mouth feel". The latter two terms relate to the flow properties of the material, such as spreadability and the ability to "melt" (transform from gel to liquid emulsion) on contact with the warmth of the mouth.
Dairy products
Milk is a far more complex food than is described here; see Dairy Chemistry and Physics (U. of Guelph) or this Wikipedia page .
Milk is basically an emulsion of lipid oils ("butterfat") dispersed in water and stabilized by phospholipids and proteins. Most of the protein content of milk consists of a group known as caseins which aggregate into a complex micellar structure which is bound together by calcium phosphate units.
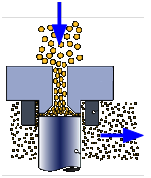
Homogenizer
The stabilizers present in fresh milk will maintain its uniformity for 12-24 hours, but after this time the butterfat globules begin to coalesce and float to the top ("creaming"). In order to retard this process, most milk sold after the early 1940's undergoes homogenization in which the oil particles are forced through a narrow space under high pressure. This breaks up the oil droplets into much smaller ones which remain suspended for the useful shelf life of the milk.
Before homogenization become common, milk bottles commonly had enlarged tops ↑
to make it easier to skim off the cream that would separate out.
The structures of cream, yogurt and ice cream are dominated by the casein aggregates mentioned above.
Ice cream is a complex mixture of several colloid types:
- an emulsion (of butterfat globules in a highly viscous aquatic phase);
- a semisolid foam consisting of small (100 μ) air bubbles which are beat into the mixture as it is frozen. Without these bubbles, the frozen mixture would be too hard to conveniently eat;
- a gel in which a network of tiny (50 μ) ice crystals are dispersed in a semi-glassy aqueous phase containing sugars and dissolved macromolecules.

Whereas milk is an oil (butterfat)-in-water dispersion, butter and margarine have a "reversed" (water-in-oil) arrangement. This transformation is accomplished by subjecting the butterfat droplets in cream to violent agitation (chur ning) which forces the droplets to coalesce into a semisolid mass within which remnants of the water phase are embedded. The greater part of this phase ends up as the by-product buttermilk.
Eggs: colloids for breakfast, lunch, and dessert

A detailed study of eggs and their many roles in cooking can amount to a mini-course in colloid chemistry in itself. There is something almost magical in the way that the clear, viscous "white" of the egg can be transformed into a white, opaque semi-solid by brief heating, or renderd into more intricate forms by poaching, frying, scrambling, or baking into custards, soufflés, and meringues, not to mention tasty omelettes, quiches, and more exotic delights such as the eggah (Arabic) and kuku (Persian) dishes of the Middle-East.
The raw eggwhite is basically a colloidal sol of long-chain protein molecules, all curled up into compact folded forms due to hydrogen bonding between different parts of the same molecule. Upon heating, these bonds are broken, allowing the proteins to unfold. The denuded chains can now tangle and bind to each other, transforming the sol into a cross-linked hydrogel, now so dense that scattered light changes its appearance to opaque white.
What happens next depends very much on the skill of the cook. The idea is to drive out enough of the water entrapped within the gel network to achieve the desired density while retaining enough gel structure to prevent it from forming a rubbery mass, as usually happens with hard-boiled eggs. This is especially important when the egg structure is to be incorporated into other food components as in baked dishes.
The key to all this is temperature control; the eggwhite proteins begin to coagulate at 65°C and if yolk proteins are present, the mixture is nicely set at about 73°; by 80° the principal (albumin) protein has set, and at much above this the gel network will collapse into an overcooked mass. The temperature limit required to avoid this disaster can be raised by adding milk or sugar; the water part of the milk dilutes the proteins, while suger molecules hydrogen-bond to them, forming a protective shield that keeps the proton strand separated. This is essential when baking custards, but incorporating a bit of cream into scrambled eggs can similarly help them retain their softness.

Whipped cream and meringues
The other colloidal personalities eggs can display are liquid and solid foams. Instead of applying heat to unfold the proteins, we "beat" them; the shearing force of a whisk or egg-beater helps pull them apart, and the air bubbles that get entrapped in the mixture attract the hydrophobic parts of the unfolded proteins and help hold them in place. Sugar will stabilize the foam by raising its viscosity, but will interfere with protein folding if added before the foam is fully formed. Sugar also binds the residual water during cooking, retarding its evaporation until after the proteins not broken up by beating can be thermally coagulated.
Paints and inks
Paints have been used since ancient times for both protective and decorative purposes. They consist basically of pigment particles dispersed in vehicle — a liquid capable for forming a stable solid film as the paint "dries".
The earliest protective coatings were made by dissolving plant-derived natural polymers (resins) in an oil such as that of linseed. The double-bonds in these oils tends to oxidize when exposed to air, causing it to polymerize into an impervious film. The colloidal pigments were stabilized with naturally-occuring surfactants such as polysaccharide gums.
Present-day paints are highly-engineered products specialized for particular industrial or architectural coatings and for marine or domestic use. For environmental reasons, water-based ("latex") vehicles are now preferred.
Inks
The most critical properties of inks relate to their drying and surface properties; they must be able to flow properly and attach to the surface without penetrating it — the latter is especially critical when printing on a porous material such as paper.
Many inks consist of organic dyes dissolved in a water-based solvent, and are not colloidal at all. The ink used in printing newspapers employs colloidal carbon black dispersed in an oil vehicle. The pressure applied by the printing press forces the vehicle into the pores of the paper, leaving most of the pigment particles on the surface.
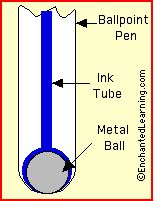 The inks employed in ball-point pens are gels, formulated in such a way that the ink will only flow over the ball and onto the paper when the shearing action of the ball (which rotates as it moves across the paper) "breaks" the gel into a liquid; the resulting liquid coats the ball and is transferred to the paper. As in conventional printing, the pigment particles remain on the paper surface, while the liquid is pressed into the pores and gradually evaporates.
The inks employed in ball-point pens are gels, formulated in such a way that the ink will only flow over the ball and onto the paper when the shearing action of the ball (which rotates as it moves across the paper) "breaks" the gel into a liquid; the resulting liquid coats the ball and is transferred to the paper. As in conventional printing, the pigment particles remain on the paper surface, while the liquid is pressed into the pores and gradually evaporates.
Colloids in water and wastewater treatment

Turbidities of 5, 50, and 500 units. [WikiMedia]
The sulfates of aluminum (alum) and of iron(III) have long been widely employed for this purpose. Synthetic polymers tailored specifically for these applications have more recently come into use.
The usual method of removing turbidity is to add a flocculating agent (flocculant). These are most often metallic salts that can form gel-like hydroxide precipitates, often with the aid of added calcium hydroxide (quicklime) if pH of the water must be raised.
 The flocculant salts neutralize the surface charges of the colloids, thus enabling them to coagulate; these are engulfed and trapped by fragments of gelatinous precipitate, which are drawn together into larger aggregates by gentle agitation until they become sufficiently large to form flocs which can be separated by settling or filtration.
The flocculant salts neutralize the surface charges of the colloids, thus enabling them to coagulate; these are engulfed and trapped by fragments of gelatinous precipitate, which are drawn together into larger aggregates by gentle agitation until they become sufficiently large to form flocs which can be separated by settling or filtration.
Colloids in soils
The four major components of soils are mineral sediments, organic matter, water, and air. The water is primarily adsorbed to the mineral- and organic- meterials, but may also share pore spaces with air; pore spaces constitute about half the bulk volume of typical soild.
The principal colloidal components of soils are mineral sediments in the form of clays, and the humic materials in the organic matter. In addition to influencing the consistency of soil by binding water molecules, soil colloids play an essential role in storing and exchanging the mineral ions required by plants.

Most soil colloids are negatively charged, and therefor attract cations such as Ca2+, Mg2+, and K+ into the outer parts of their double layers. Because these ions are loosely bound, they constitute a source from which plant roots can draw these essential nutrients. Conversely, they can serve as a sink for these same ions when they are released after the plant dies.
Clay colloids
Clays are layered structures based on alumino-silicates or hydrous oxides, mostly of iron or aluminum. Each layer is built of two or three sheets of extended silica or alumina structures linked together by shared oxygen atoms. These layers generally have an overall negative charge owing to the occasional replacement of a Si4+ ion by one of Al3+.
Adjacent layers are separated by a region of adsorbed cations (to neutralize the negative charges) and water molecules, and thus are held together relatively loosely. It is these interlayer regions that enable clays to work their magic by exchanging ions with both the soil water and the roots of plants.
Humic substances
The principal organic components of soil are complex substances of indeterminate structure that present –OH and –COOH groups which become increasingly dissociated as the pH increases. This allows them to bind and exchange cations in much the same way as described above.
Make sure you thoroughly understand the following essential concepts that have been presented above.
- Summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions.
- For the various dispersion types (emulsion, gel, sol, foam, etc.), name the type (gas, liquid, or solid) of both the dispersed phase and the dispersions phase.
- Describe the origins of Brownian motion and how it can observed.
- Describe the electric double layer that surrounds many colloidal particles.
- Explain the mechanisms responsible for the stability of lyophilic and lyophobic colloidal dispersions.
- Define: surfactant, detergent, emulsifier, micelle.
- Give some examples of how colloidal dispersions can be made.
- Explain why freezing or addition of an electrolyte can result in the coagulation of an emulsion.
- Describe some of the colloid-related principles involved in food chemistry, such as the stabilization of milk and mayonaise, the preparation of butter, and the various ways of cooking eggs.
- Describe the role of colloids in wastewater treatment.

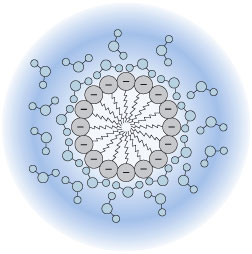
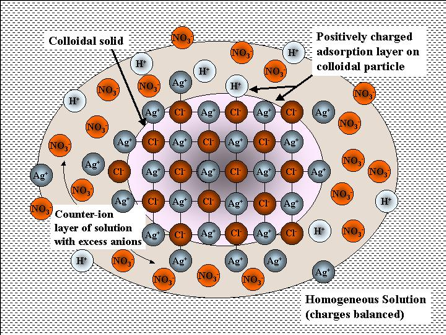 Representation of a colloidal sol of silver chloride and its electric double layer. The tiny crystal of AgCl is shown in the inner circle. The [larger] chloride ions near the surface adsorb cations from the solution, giving the particle a net positive charge. This charge is balanced by a slight excess of anions in the outer circle.
Representation of a colloidal sol of silver chloride and its electric double layer. The tiny crystal of AgCl is shown in the inner circle. The [larger] chloride ions near the surface adsorb cations from the solution, giving the particle a net positive charge. This charge is balanced by a slight excess of anions in the outer circle. 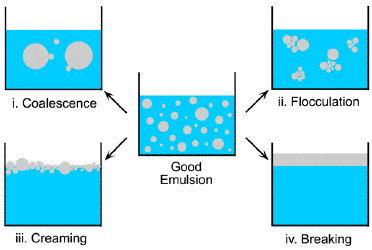
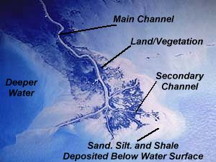 Rivers carry millions of tons of colloidal clay into the oceans. If you fly over the mouth of a river such as the Missippipi (shown here in a satellite image), you can sometimes see the difference in color as the clay colloids coagulate due to the action of the salt water.
Rivers carry millions of tons of colloidal clay into the oceans. If you fly over the mouth of a river such as the Missippipi (shown here in a satellite image), you can sometimes see the difference in color as the clay colloids coagulate due to the action of the salt water.