 We begin with the image you saw in the preceding lesson, showing the long form of the table with the "block" structure emphasized. You will recall that the two f blocks are written at the bottom merely to keep the table from becoming inconveniently wide; these two blocks actually go in between La-Hf and Ac-Db, respectively, in the d block.
We begin with the image you saw in the preceding lesson, showing the long form of the table with the "block" structure emphasized. You will recall that the two f blocks are written at the bottom merely to keep the table from becoming inconveniently wide; these two blocks actually go in between La-Hf and Ac-Db, respectively, in the d block.
A very simple way of organizing the chemical elements is to make a long a long horizontal list of the elements in order of their increasing atomic number. It would begin this way:
H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca...
Now if we look at the various physical and chemical properties of these elements, we would find that their values tend to increase or decrease with Z in a manner that reveals a repeating pattern— that is, a periodicity. For the elements listed above, these breaks can be indicated by the vertical bars shown here in color:
H He | Li Be B C N O F Ne | Na Mg Al Si P S Cl Ar | Ca ...
Periods, groups and blocks
To construct the table, we place each sequence (denoted by the vertical red bar above) in a separate row, which we call a period. The rows are aligned in such a way that the elements in each vertical column possess certain similarities. Thus the first short-period elements H and He are chemically similar to the elements Li and Ne at the beginning and end of the second period. Notice that the first period is split in order to reflect these chemical similarities.

Take a moment to see how the above image relates to the complete periodic table, which we reproduce below. Each row that begins with H down through Fr corresponds to a period; in this table, there are seven periods.
Each column, labeled with small blue numbers 1-18 along the top of the table corresponds to a group.
In the past, two different systems of Roman numerals and letters were used to denote the various groups. North Americans added the letter B to denote the d-block groups and A for the others; this is the system shown in the table above. But the rest of the world used A for the d-block elements and B for the others.
In 1985, a new international system was adopted in which the columns were simply labeled 1-18. Although this system was initially resisted by North American chemists who predicted that it would leave students hopelessly confused, the "one to eighteen" system gradually became accepted as the older chemists died off and the Americans belatedly cought up with the rest of the world.

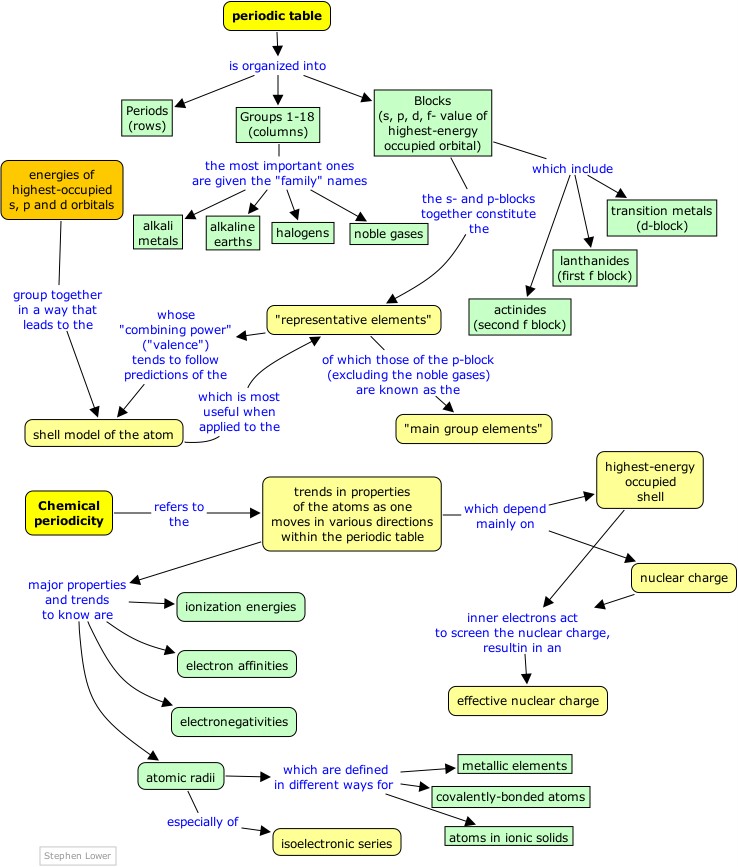
Now look at the section of the table containing groups 3 through 12, labeled
d-block. At the bottom of Group 3 in this block, notice the elements Lanthanum and Actinium, which are shaded in light green. You will see a downward-pointing arrow that points to rwo more rows, also shaded in green.
These two f-blocks, as they are called, are not considered separate periods in this "short form" of the table, but have been squeezed in, beginning at the third period, where Scandium Sc is the first element at which the 3d shell begins to fill.
The "block" nomenclature of the periodic table refers to the sub-orbital type (quantum number ℓ, or s-p-d-f classification) of the highest-energy orbitals that are occupied in a given element. Notice that:
- For n=1 there is no p block, and the s block is split so that helium is placed in the same group as the other inert gases, which it resembles chemically.
- For the second period (n=2) there is a p block but no d block; in the usual "long form" of the periodic table it is customary to leave a gap between these two blocks in order to accommodate the d blocks that occur at n=3 and above.
- At n=6 we introduce an f block, but in order to hold the table to reasonable dimensions the f blocks are placed below the main body of the table.
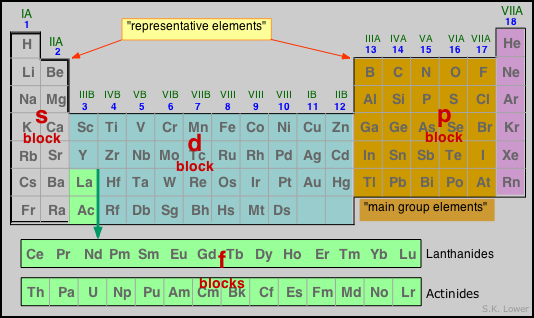
Periodic table families
Chemists have long found it convenient to refer to certain catagories of elements by the by special names, commonly known as families. The labels superimposed on the periodic table below are widely used, and worth knowing.
Of the families shown in the image, it is particularly important that you be able to recognize the alkali metals which begin each row, transition metals (all elements in the d-block Groups 3-12) and the noble gases of Group 18; these families relate directly to the electron configurations of the elements.
Other element families can be entirely arbitrary, such as the elements present in living organisms, the precious or coinage metals, the structural metals such as iron, aluminum and titanium, the elements that are commercially mined in a given country, etc.
The properties of an atom depend ultimately on the number of electrons in the various orbitals, and on the nuclear charge which determines the compactness of the orbitals.
The shell model of the atom
In order to relate the properties of the elements to their locations in the periodic table, it is often convenient to make use of a simplified view of the atom in which the nucleus is surrounded by one or more concentric spherical "shells", each of which consists of the highest-principal quantum number orbitals (always s- and p-orbitals) that contain at least one electron.
As with any scientific model, the shell model offers a simplified view that helps us to understand and correlate diverse phenomena. The principal simplification here is that it deals only with the main group elements of the s- and p-blocks, omitting the d- and f-block elements whose properties tend to be less closely tied to their group numbers.
In particular, the number of outer-shell electrons (which is given by the rightmost digit in the group number) is a major determinant of an element's "combining power", or valence. The general trend is for an atom to gain or lose electrons, either directly (leading to formation of ions) or by sharing electrons with other atoms so as to achieve an outer-shell configuration of s2p6. This configuration, known as an octet, corresponds to that of one of the noble-gas elements of Group 18.
- the elements in Groups 1, 2 and 13 tend to give up their valence electrons to form positive ions such as Na+, Mg2+ and Al3+, as well as compounds NaH, MgH2 and AlH3. The outer-shell configurations of the metal atoms in these species correspond to that of neon.
- elements in Groups 15-17 tend to acquire electrons, forming ions such as P3–, S2– and Cl– or compounds such as PH3, H2S and HCl. The outer-shell configurations of these elements correspond to that of argon.
- the Group 14 elements do not normally form ions at all, but share electrons with other elements in tetravalent compounds such as CH4.
Effective nuclear charge
Those electrons in the outmost or valence shell are especially important because they are the ones that can engage in the sharing and exchange that is responsible for chemical reactions; how tightly they are bound to the atom determines much of the chemistry of the element. The degree of binding is the result of two opposing forces: the attraction between the electron and the nucleus, and the repulsions between the electron in question and all the other electrons in the atom. All that matters is the net force, the difference between the nuclear attraction and the totality of the electron-electron repulsions.
We can simplify the shell model even further by imagining that the valence shell electrons are the only electrons in the atom, and that the nuclear charge has whatever value would be required to bind these electrons as tightly as is observed experimentally. Because the number of electrons in this model is less than the atomic number Z, the required nuclear charge will also be smaller, and is known as the effective nuclear charge. Effective nuclear charge is essentially the positive charge that a valence electron "sees".
Part of the difference between Z and Zeffective is due to other electrons in the valence shell, but this is usually only a minor contributor because these electrons tend to act as if they are spread out in a diffuse spherical shell of larger radius. The main actors here are the electrons in the much more compact inner shells which surround the nucleus and exert what is often called a shielding or "screening" effect on the valence electrons.
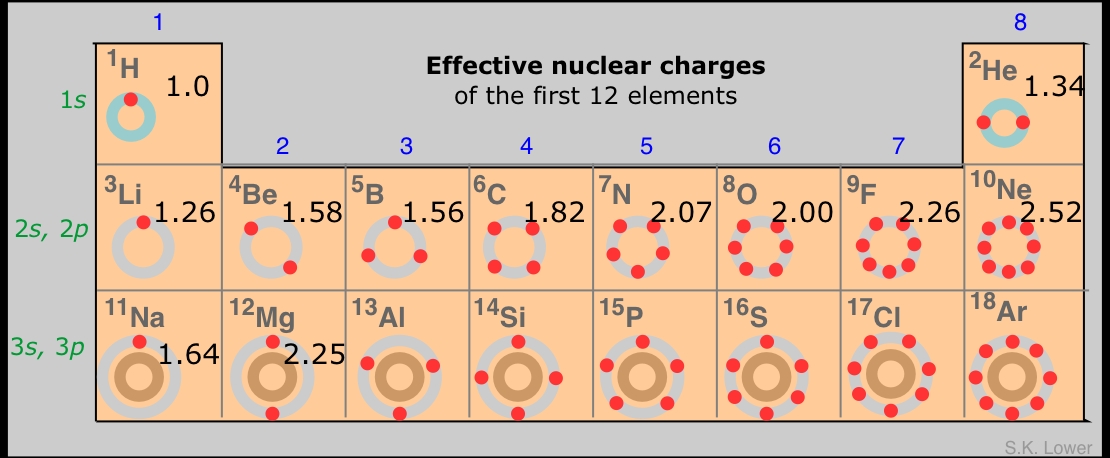
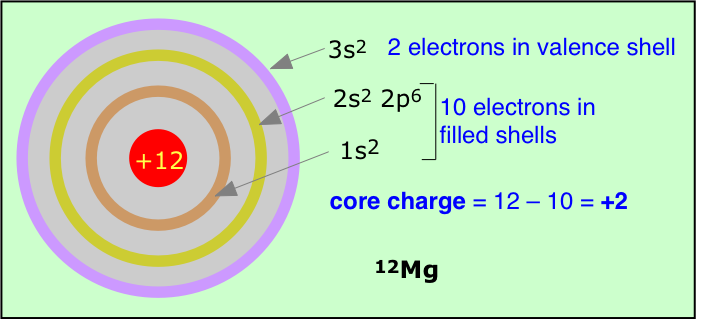
The formula for calculating effective nuclear charge is not very complicated, but we will skip a discussion of it here. An even simpler although rather crude procedure is to just subtract the number of inner-shell electrons from the nuclear charge; the result is a form of effective nuclear charge which is called the core charge of the atom.
The original purpose of the periodic table was to organize the the chemical elements in a manner that would make sense of the ways in which the oobserved physical and chemical properties of the elements vary with the atomic number.
In this section, we look at some of these trends and try to understand the reasons for them based on the electron structures of the elements.
Periodic trends in the sizes of atoms
What do we mean by the "size" of an atom?
When an atom is combined with other atoms in a solid element or compound, an effective radius can be determined by observing the distances between adjacent rows of atoms in these solids. This is most commonly carried out by X-ray scattering experiments. Because of the different ways in which atoms can aggregate together, several different kinds of atomic radii can be defined.
Distances on the atomic scale have traditionally been expressed in Ångstrom units (1Å = 10–8 cm = 10–10 m), but nowadays the picometer is preferred;
1 pm = 10–12 m = 10–10 cm = 10–2 Å, or 1Å = 100 pm. The radii of atoms and ions are typically in the range 70-400 pm.
A rough idea of the size of a metallic atom can be obtained simply by measuring the density of a sample of the metal. This tells us the number of atoms per unit volume of the solid. The atoms are assumed to be spheres of radius r in contact with each other, each of which sits in a cubic box of edge length 2r. The volume of each box is just the total volume of the solid divided by the number of atoms in that mass of the solid; the atomic radius is the cube root of r.
Although the radius of an atom or ion cannot be measured directly, in most cases it can be inferred from measurements of the distance between adjacent nuclei in a crystalline solid.
Because solids fall into several different classes, several kinds of atomic radius are defined. Many atoms have several different radii; for example,
sodium forms a metallic solid and thus has a metallic radius, it forms a gaseous molecule Na2 in the vapor phase (covalent radius), and of course it forms ionic solids such as NaCl.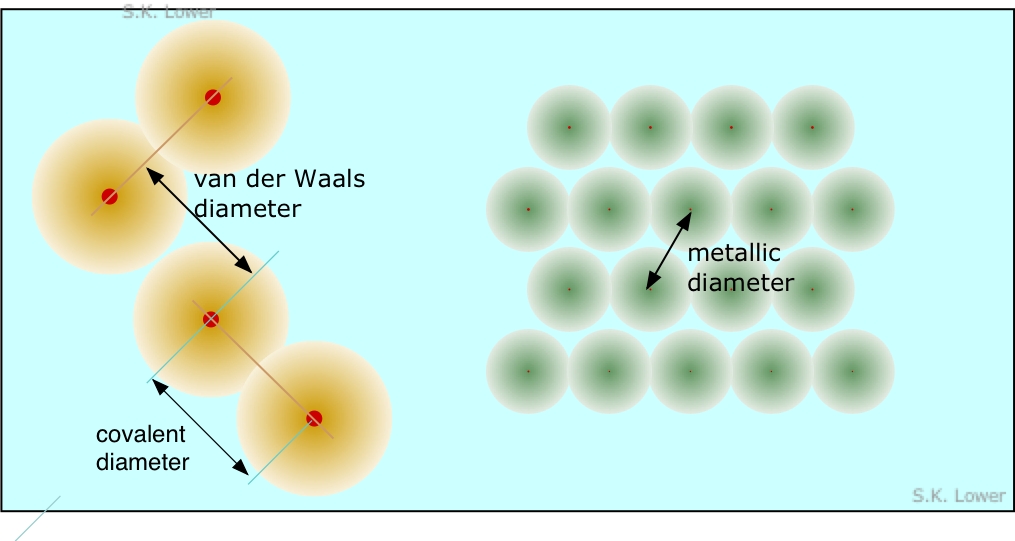
- Metallic radius is half the distance between nuclei in a metallic crystal.
- Covalent radius is half the distance between like atoms that are bonded together in a molecule.
- van der Waals radius is the effective radius of adjacent atoms which are not chemically bonded in a solid, but are presumably in "contact". An example would be the distance between the iodine atoms of adjacent I2 molecules in crystalline iodine.
Many atoms have several different radii; for example, sodium forms a metallic solid and thus has a metallic radius, it forms a gaseous molecule Na2 in the vapor phase (covalent radius), and of course it forms ionic solids as mentioned above.
Periodic trends in atomic radius
We would expect the size of an atom to depend mainly on the principal quantum number of the highest occupied orbital; in other words, on the "number of occupied electron shells". Since each row in the periodic table corresponds to an increment in n, atomic radius increases as we move down a column. The other important factor is the nuclear charge; the higher the atomic number, the more strongly will the electrons be drawn toward the nucleus, and the smaller the atom. This effect is responsible for the contraction we observe as we move across the periodic table from left to right.
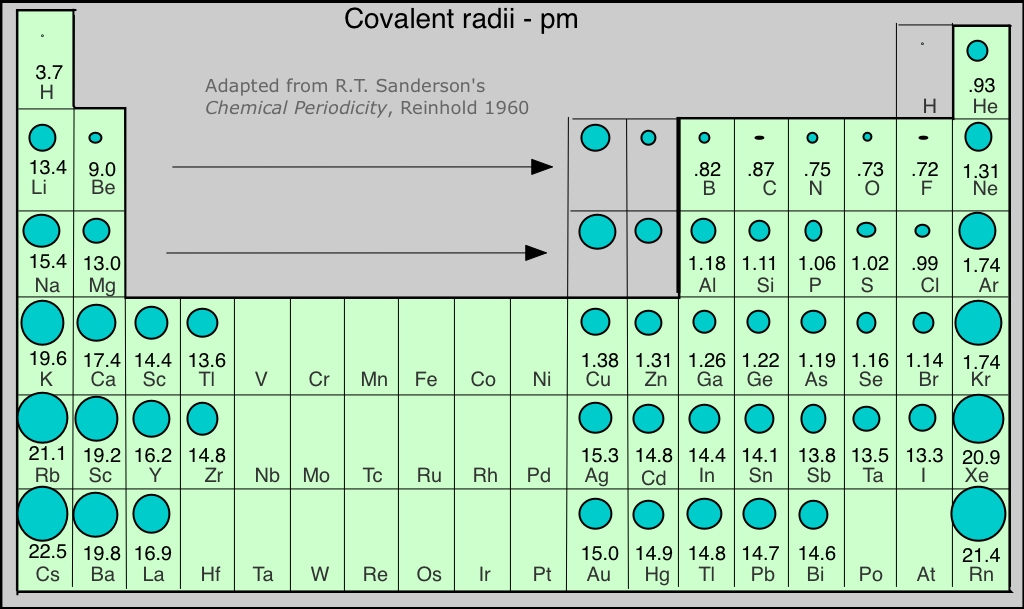
The figure shows a periodic table in which the sizes of the atoms are represented graphically. The apparent discontinuities in this diagram reflect the difficulty of comparing the radii of atoms of metallic and nonmetallic bonding types. Radii of the noble gas elements are estimates from those of nearby elements.
How ionic radii are estimated
The size of an ion can be defined only for those present in ionic solids. When an ion dissolves in water, it acquires a hydration shell of loosely-attached H2O molecules that increase its effective radius in ways that are difficult to define in a systematic way.
By observing the diffraction of X-rays by an ionic crystal, it is an easy task to measure the distance between adjacent rows of Na+ and Cl– ions, but there is no unambiguous way to decide what portions of this distance are attributable to each ion. The best one can do is make estimates based on studies of several different ionic solids (LiI, KI, NaI, for example) that contain one ion in common. Many such estimates have been made, and they turn out to be remarkably consistent.
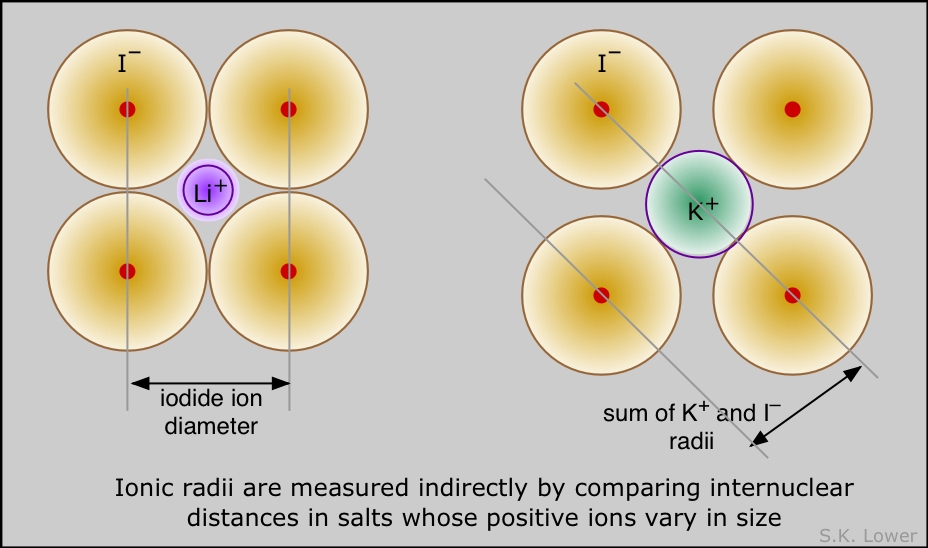
Comparing the radii of atoms and their ions
A positive ion is always smaller than the neutral atom, owing to the diminished electron-electron repulsion. If a second electron is lost, the ion gets even smaller; for example, the ionic radius of Fe2+ is 76 pm, while that of Fe3+ is 65 pm. If formation of the ion involves complete emptying of the outer shell, then the decrease in radius is especially great.
The hydrogen ion H+ is in a class by itself; having no electron cloud at all, its radius is that of the bare proton, or about 0.1 pm— a contraction of 99.999%! Because the unit positive charge is concentrated into such a small volume of space, the charge density of the hydrogen ion is extremely high; it interacts very strongly with other matter, including water molecules, and in aqueous solution it exists only as the hydronium ion H3O+.
Negative ions are always larger than the parent ion; the addition of one or more electrons to an existing shell increases electron-electron repulsion which results in a general expansion of the atom.
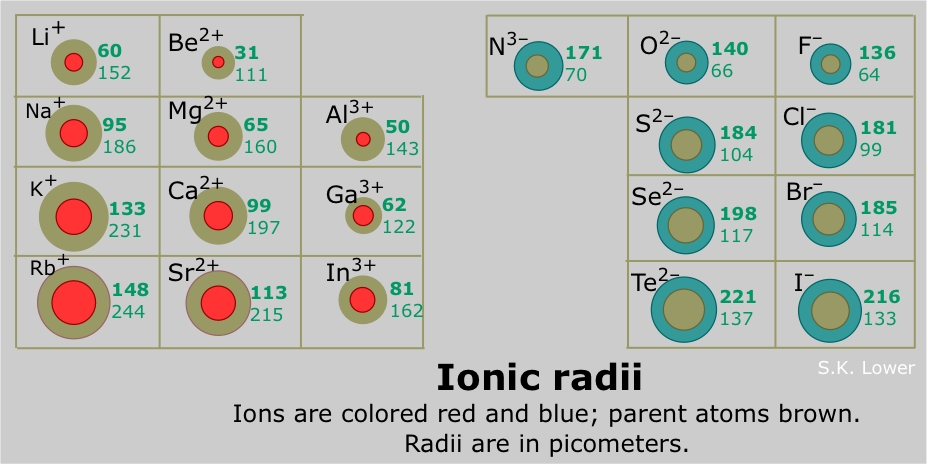
Periodic trends in ionic radii
The best way of visualizing these trends is to compare the radii of an isoelectronic series — a sequence of species all having the same number of electrons (and thus the same amount of electron-electron repulsion) but differing in nuclear charge. Of course, only one member of such a sequence can be a neutral atom (neon in the series shown below.) The effect of increasing nuclear charge on the radius is clearly seen.
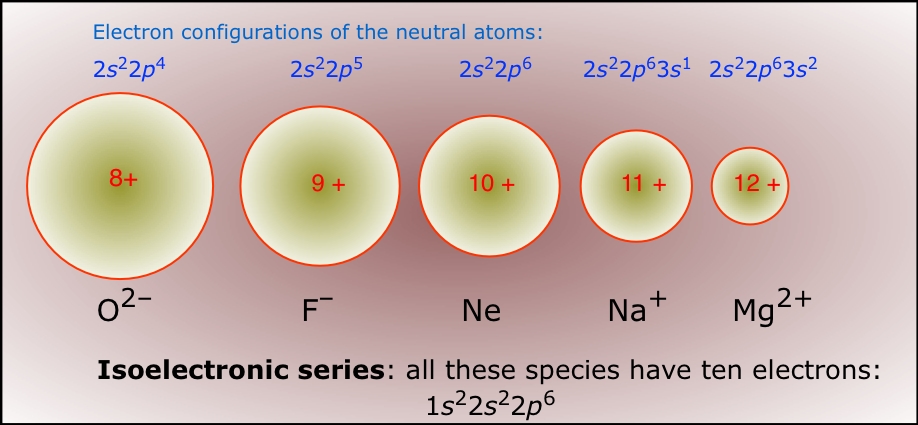
Chemical reactions are based largely on the interactions between the most loosely bound electrons in atoms, so it is not surprising that the tendency of an atom to gain, lose or share electrons should play an important role in determining its chemical properties.
Periodic trends in ionization energy
The term "ionization energy" always refers to removal of electrons of an atom, leading to the formation of positive ions. In order to remove an electron from an atom, work must be done to overcome the electrostatic attraction between the electron and the nucleus; this work is called the ionization energy of the atom and corresponds to the exothermic process
M(g) → M+(g) + e–
in which M(g) stands for any isolated (gaseous) atom.
An atom has as many ionization energies as it has electrons. Electrons are always removed from the highest-energy occupied orbital. An examination of the successive ionization energies of the first ten elements (below) provides experimental confirmation that the binding of the two innermost electrons (1s orbital) is significantly different from that of the n=2 electrons.Successive ionization energies of an atom increase rapidly as reduced electron-electron repulsion causes the electron shells to contract, thus binding the electrons even more tightly to the nucleus.
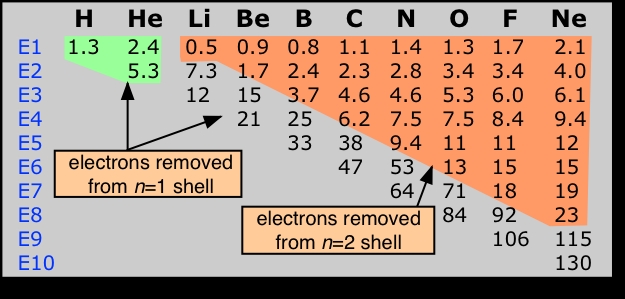
Ionization energies increase with the nuclear charge Z as we move across the periodic table. They decrease as we move down the table because in each period the electron is being removed from a shell one step farther from the nucleus than in the atom immediately above it. This results in the familiar zig-zag lines when the first ionization energies are plotted as a function of Z.
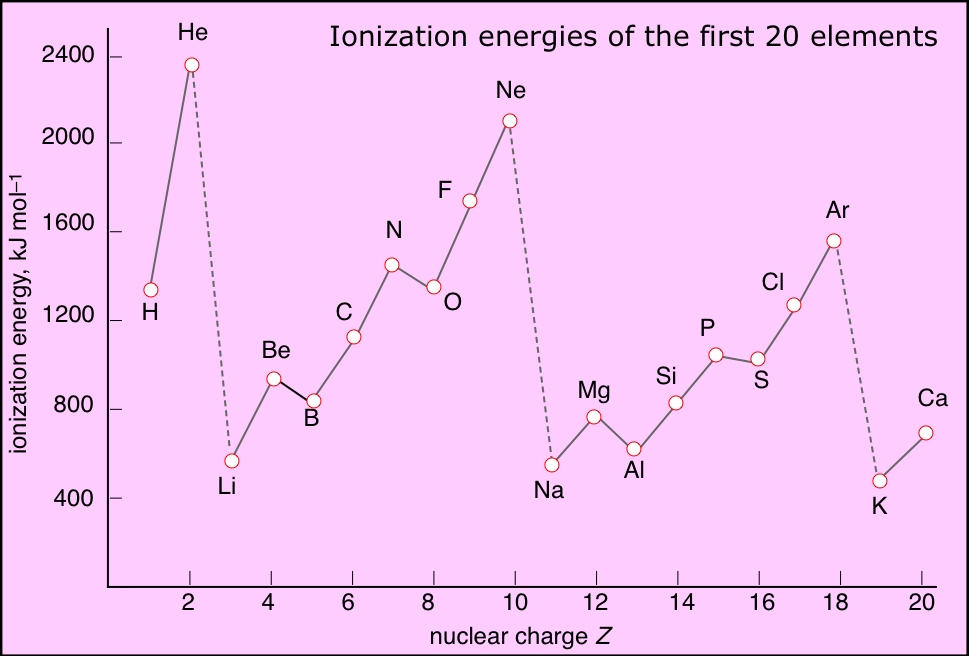
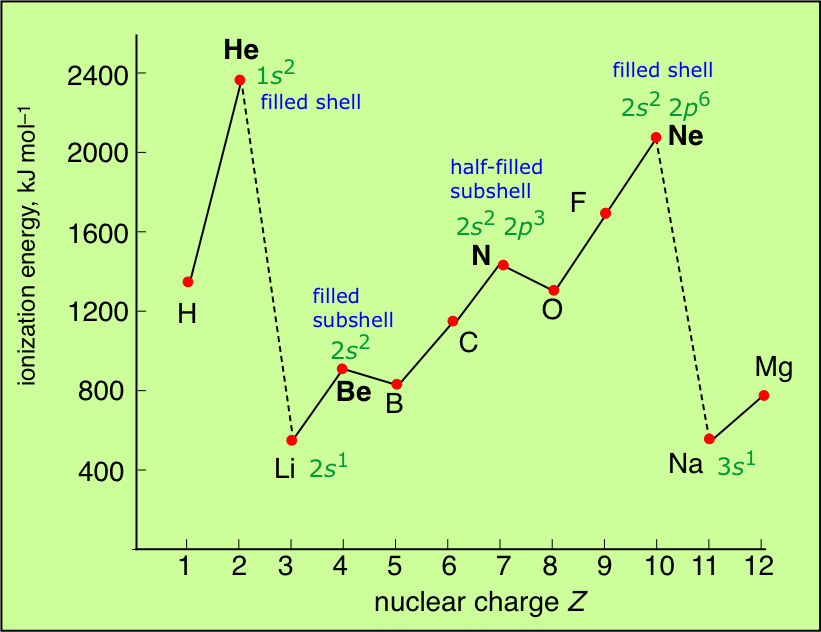
Depicting the ionization energies of the main group elements in the context of the periodic table offers a more comprehensive view of these trands.
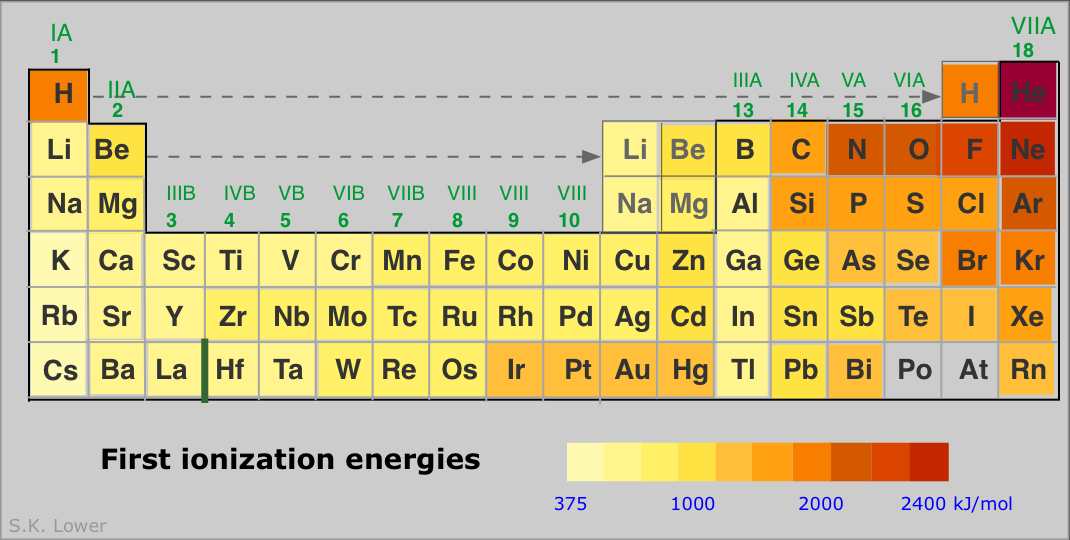
Some points to note:
- The noble gases have the highest IE's of any element in the period. This has nothing to do with any mysterious "special stability" of the s2p6 electron configuration; it is simply a matter of the high nuclear charge acting on more contracted orbitals.
- IE's (as well as many other properties) tend not to vary greatly amongst the d-block elements. This reflects the fact that as the more-compact d orbitals are being filled, they exert a screening effect that partly offsets that increasing nuclear charge on the outermost s orbitals of higher principal quantum number.
- Each of the Group 13 elements has a lower first-IE than that of the element preceding it. The reversal of the IE trend in this group is often attributed to the more easy removal of the single outer-shell p electron compared to that of electrons contained in filled (and thus spin-paired) s- and d-orbitals in the preceding elements.
Periodic trends in electron affinity
Formation of a negative ion occurs when an electron from some external source enters the atom and become incorporated into the lowest energy orbital that possesses a vacancy. Because the entering electron is attracted to the positive nucleus, the formation of negative ions is usually exothermic. The energy given off is the electron affinity of the atom. For some atoms, the electron affinity appears to be slightly negative, suggesting that electron-electron repulsion is the dominant factor in these instances.
In general, electron affinities tend to be much smaller than ionization energies, suggesting that they are controlled by opposing factors having similar magnitudes. These two factors are, as before, the nuclear charge and electron-electron repulsion. But the latter, only a minor actor in positive ion formation, is now much more significant. One reason for this is that the electrons contained in the inner shells of the atom exert a collective negative charge that partially cancels the charge of the nucleus, thus exerting a so-called shielding effect which diminishes the tendency for negative ions to form.
Because of these opposing effects, the periodic trends in electron affinities are not as clear as are those of ionization energies. This is particularly evident in the first few rows of the periodic table, in which small effects tend to be magnified anyway because an added electron produces a large percentage increase in the number of electrons in the atom.
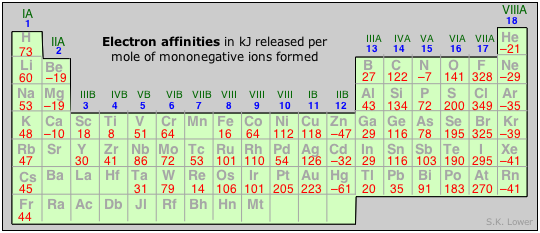
In general, we can say that electron affinities become more exothermic as we move from left to right across a period (owing to increased nuclear charge and smaller atom size). There are some interesting irregularities, however:
- In the Group 2 elements, the filled 2s orbital apparently shields the nucleus so effectively that the electron affinities are slightly endothermic.
- The Group 15 elements have rather low values, due possibly to the need to place the added electron in a half-filled p orbital; why the electron affinity of nitrogen should be endothermic is not clear. The vertical trend is for electron affinity to become less exothermic in successive periods owing to better shielding of the nucleus by more inner shells and the greater size of the atom, but here also there are some apparent anomalies.
Electronegativity
When two elements are joined in a chemical bond, the element that attracts the shared electrons more strongly is more electronegative. Elements with low electronegativities (the metallic elements) are said to be electropositive.
Moreover, the same atom can exhibit different electronegativities in different chemical environments, so the "electronegativity of an element" is only a general guide to its chemical behavior rather than an exact specification of its behavior in a particular compound. Nevertheless, electronegativity is eminently useful in summarizing the chemical behavior of an element. You will make considerable use of electronegativity when you study chemical bonding and the chemistry of the individual elements.
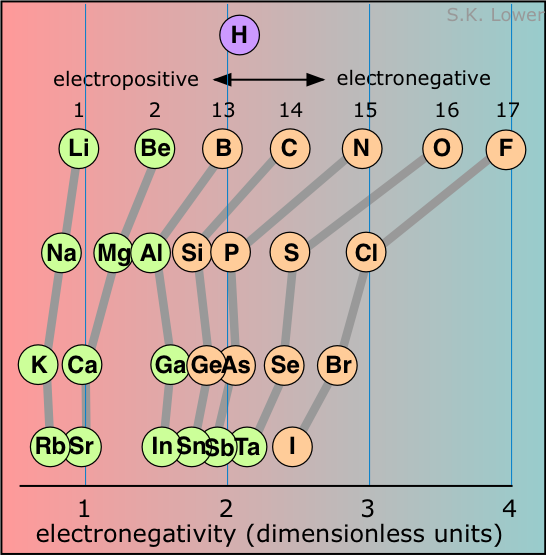 Because there is no single definition of electronegativity, any numerical scale for measuring it must of necessity be somewhat arbitrary. Most such scales are themselves based on atomic properties that are directly measurable and which relate in one way or the other to electron-attracting propensity.
Because there is no single definition of electronegativity, any numerical scale for measuring it must of necessity be somewhat arbitrary. Most such scales are themselves based on atomic properties that are directly measurable and which relate in one way or the other to electron-attracting propensity.
The most widely used of these scales was devised by Linus Pauling and is related to ionization energy and electron affinity.
The Pauling scale runs from 0 to 4; the highest electron affinity, 4.0, is assigned to fluorine, while cesium has the lowest value of 0.7. Values less than about 2.2 are usually associated with electropositive, or metallic character. In the representation of the scale shown in figure, the elements are arranged in rows corresponding to their locations in the periodic table. The correlation is obvious; electronegativity is associated with the higher rows and the rightmost columns.
The location of hydrogen on this scale reflects some of the significant chemical properties of this element. Although it acts like a metallic element in many respects (forming a positive ion, for example), it can also form hydride-ion (H–) solids with the more electropositive elements, and of course its ability to share electrons with carbon and other p-block elements gives rise to a very rich chemistry, including, of course, the millions of organic compounds.