The Le Châtelier Principle
Shifting the equilibrium
The previous lesson emphasized the dynamic character of equilibrium as expressed by the Law of Mass Action. This law serves as a model explaining how the composition of the equilibrium state is affected by the "active masses" (concentrations) of reactants and products.
In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is affected by the addition or withdrawal of one of the components.
 If a reaction is at equilibrium and we alter the conditions so as to create a new equilibrium state, then the composition of the system will tend to change until that new equilibrium state is attained. (We say "tend to change" because if the reaction is kinetically inhibited, the change may be too slow to observe or it may never take place.) In 1884, the French chemical engineer and teacher Henri Le Châtelier (1850-1936) showed that in every such case, the new equilibrium state is one that partially reduces the effect of the change that brought it about.
If a reaction is at equilibrium and we alter the conditions so as to create a new equilibrium state, then the composition of the system will tend to change until that new equilibrium state is attained. (We say "tend to change" because if the reaction is kinetically inhibited, the change may be too slow to observe or it may never take place.) In 1884, the French chemical engineer and teacher Henri Le Châtelier (1850-1936) showed that in every such case, the new equilibrium state is one that partially reduces the effect of the change that brought it about.
This law is known to every Chemistry student as the Le Châtelier principle. His original formulation was somewhat complicated, but a reasonably useful paraphrase of it reads as follows:
To see how this works (and you must do so, as this is of such fundamental importance that you simply cannot do any meaningful chemistry without a thorough working understanding of this principle), look again at the hydrogen iodide dissociation reaction
2 HI → H2 + I2
Consider an arbitrary mixture of these three components at equilibrium, and assume that we inject more hydrogen gas into the container. Because the H2 concentration now exceeds its initial equilibrium value, the system is no longer in its equilibrium state, so a net reaction now ensues as the system moves to the new state.
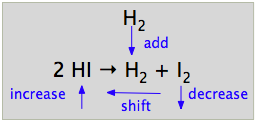 The Le Châtelier principle states that the net reaction will be in a direction that tends to reduce the effect of the added H2. This can occur if some of the H2 is consumed by reacting with I2 to form more HI; in other words, a net reaction occurs in the reverse direction. Chemists usually simply say that "the equilibrium shifts to the left".
The Le Châtelier principle states that the net reaction will be in a direction that tends to reduce the effect of the added H2. This can occur if some of the H2 is consumed by reacting with I2 to form more HI; in other words, a net reaction occurs in the reverse direction. Chemists usually simply say that "the equilibrium shifts to the left".
The following table contains several examples showing how changing the quantity of a reaction component can shift an established equilibrium.
| System | Change | Result |
|---|---|---|
| CO2 + H2 → H2O(g) + CO | a drying agent is added to absorb H2O | Shift to the right. Continuous removal of a product will force any reaction to the right |
| H2(g)+ I2(g) → 2HI(g) | Some nitrogen gas is added | No change; N2 is not a component of this reaction system. (Recall Dalton's law: "Every gas is a vacuum to every other gas".) |
| NaCl(s)+ H2SO4(l) → Na2SO4(s) + HCl(g) | reaction is carried out in an open container | Because HCl is a gas that can escape from the system, the reaction is forced to the right. This is the basis for the commercial production of hydrochloric acid. |
| H2O(l) → H2O(g) | water evaporates from an open container | Continuous removal of water vapor forces the reaction to the right, so equilibrium is never achieved. |
| HCN(aq) → H+(aq)+ CN–(aq) | the solution is diluted | Shift to right; the product [H+][CN—] diminishes more rapidly than does [HCN]. |
| AgCl(s) → Ag+(aq)+ Cl–(aq) | some NaCl is added to the solution | Shift to left due to increase in Cl– concentration. This is known as the common ion effect on solubility. |
| N2(g) + 3 H2(g) → 2 NH3(g) | a catalyst is added to speed up this reaction | No change. Catalysts affect only the rate of a reaction; the have no effect at all on the composition of the equilibrium state. |
Virtually all chemical reactions are accompanied by the liberation or uptake of heat. If we regard heat as a "reactant" or "product" in an endothermic or exothermic reaction respectively, we can use the Le Châtelier principle to predict the direction in which an increase or decrease in temperature will shift the equilibrium state. Thus for the oxidation of nitrogen, an endothermic process, we can write
[heat] + N2(g) + O2(g) → 2 NO(g)
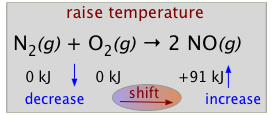 Suppose this reaction is at equilibrium at some temperature T1 and we raise the temperature to T2. The Le Châtelier principle tells us that a net reaction will occur in the direction that will partially counteract this change. Since the reaction is endothermic, a shift of the equilibrium to the right will take place.
Suppose this reaction is at equilibrium at some temperature T1 and we raise the temperature to T2. The Le Châtelier principle tells us that a net reaction will occur in the direction that will partially counteract this change. Since the reaction is endothermic, a shift of the equilibrium to the right will take place.
Nitric oxide, the product of this reaction, is a major air pollutant which initiates a sequence of steps leading to the formation of atmospheric smog. Its formation is an unwanted side reaction which occurs when the air (which is introduced into the combustion chamber of an engine to supply oxygen) gets heated to a high temperature. Designers of internal combustion engines now try, by various means, to limit the temperature in the combustion region, or to restrict its highest-temperature part to a small volume within the combustion chamber.
You will recall that if the pressure of a gas is reduced, its volume will increase; pressure and volume are inversely proportional. With this in mind, suppose that the reaction
2 NO2(g) → N2O4(g)
is in equilibrium at some arbitrary temperature and pressure, and that we double the pressure, perhaps by compressing the mixture to a smaller volume. From the Le Châtelier principle we know that the equilibrium state will change to one that tends to counteract the increase in pressure. This can occur if some of the NO2 reacts to form more of the dinitrogen tetroxide, since two moles of gas are being removed from the system for every mole of N2O4 formed, thereby decreasing the total volume of the system. Thus increasing the pressure will shift this equilibrium to the right.
For the above reaction, this change
Δng = (nproducts – nreactants) = 1 – 2 = –1.
In the case of the nitrogen oxidation reaction N2 + O2 → 2 NO,
Δng = 0 and pressure will have no effect.
The volumes of solids and liquids are hardly affected by the pressure at all, so for reactions that do not involve gaseous substances, the effects of pressure changes are ordinarily negligible. Exceptions arise under conditions of very high pressure such as exist in the interior of the Earth or near the bottom of the ocean. A good example is the dissolution of calcium carbonate
CaCO3(s) → Ca2+ + CO32–. There is a slight decrease in the volume when this reaction takes place, so an increase in the pressure will shift the equilibrium to the right, with the results that calcium carbonate becomes more soluble at higher pressures.
The skeletons of several varieties of microscopic organisms that inhabit the top of the ocean are made of CaCO3, so there is a continual rain of this substance toward the bottom of the ocean as these organisms die. As a consequence, the floor of the Atlantic ocean is covered with a blanket of calcium carbonate. This is not true for the Pacific ocean, which is deeper; once the skeletons fall below a certain depth, the higher pressure causes them to dissolve. Some of the seamounts (undersea mountains) in the Pacific extend above the solubility boundary so that their upper parts are covered with CaCO3 sediments.
The effect of pressure on a reaction involving substances whose boiling points fall within the range of commonly encountered temperature will be sensitive to the states of these substances at the temperature of interest.
For reactions involving gases, only changes in the partial pressures of those gases directly involved in the reaction are important; the presence of other gases has no effect.
The Haber process for the synthesis of ammonia is based on the exothermic reaction
N2(g) + 3 H2(g) → 2 NH3(g) ΔH = –92 kJ/mol
The Le Châtelier principle tells us that in order to maximize the amount of product in the reaction mixture, it should be carried out at high pressure and low temperature. However, the lower the temperature, the slower the reaction (this is true of virtually all chemical reactions.) As long as the choice had to be made between a low yield of ammonia quickly or a high yield over a long period of time, this reaction was infeasible economically.
Nitrogen is available for free, being the major component of air, but the strong triple bond in N2 makes it extremely difficult to incorporate this element into species such as NO3– and NH4+ which serve as the starting points for the wide variety of nitrogen-containing compounds that are essential for modern industry. This conversion is known as nitrogen fixation, and because nitrogen is an essential plant nutrient, modern intensive agriculture is utterly dependent on huge amounts of fixed nitrogen in the form of fertilizer. Until around 1900, the major source of fixed nitrogen was the NaNO3 (Chile saltpeter) found in extensive deposits in South America. Several chemical processes for obtaining nitrogen compounds were developed in the early 1900's, but they proved too inefficient to meet the increasing demand.
 Although the direct synthesis of ammonia from its elements had been known for some time, the yield of product was too small to make the process commercially feasible. In 1905, Fritz Haber (1868-1934) began to study this reaction, employing the thinking initiated by Le Châtelier and others, and the newly-developing field of thermodynamics that served as the basis of these principles. From the Le Châtelier law alone, it is apparent that this exothermic reaction is favored by low temperature and high pressure. However, it was not as simple as that: the rate of any reaction increases with the temperature, so working with temperature alone, one has the choice between a high product yield achieved only very slowly, or a very low yield quickly. Further, the equipment and the high-strength alloy steels need to build it did not exist at the time. Haber solved the first problem by developing a catalyst that would greatly speed up the reaction at lower temperatures.
Although the direct synthesis of ammonia from its elements had been known for some time, the yield of product was too small to make the process commercially feasible. In 1905, Fritz Haber (1868-1934) began to study this reaction, employing the thinking initiated by Le Châtelier and others, and the newly-developing field of thermodynamics that served as the basis of these principles. From the Le Châtelier law alone, it is apparent that this exothermic reaction is favored by low temperature and high pressure. However, it was not as simple as that: the rate of any reaction increases with the temperature, so working with temperature alone, one has the choice between a high product yield achieved only very slowly, or a very low yield quickly. Further, the equipment and the high-strength alloy steels need to build it did not exist at the time. Haber solved the first problem by developing a catalyst that would greatly speed up the reaction at lower temperatures.
 The second problem, and the development of an efficient way of producing hydrogen, would delay the practical implementation of the process until 1913, when the first plant based on the Haber-Bosch process (as it is more properly known, Carl Bosch being the person who solved the major engineering problems) came into operation. The timing could not have been better for Germany, since this country was about to enter the First World War, and the Allies had established a navel blockade of South America, cutting off the supply of nitrate for the the German munitions industry.
The second problem, and the development of an efficient way of producing hydrogen, would delay the practical implementation of the process until 1913, when the first plant based on the Haber-Bosch process (as it is more properly known, Carl Bosch being the person who solved the major engineering problems) came into operation. The timing could not have been better for Germany, since this country was about to enter the First World War, and the Allies had established a navel blockade of South America, cutting off the supply of nitrate for the the German munitions industry.
Bosch's plant operated the ammonia reactor at 200 atm and 550°C. Later, when stronger alloy steels had been developed, pressures of 800-1000 atm became common. The source of hydrogen in modern plants is usually natural gas, which is mostly methane:
| CH4 + H2O → CO + 3 H2 | formation of "synthesis gas" from methane |
| CO + H2O → CO2 + H2 | "shift reaction" carried out in reformer |
Much has been written about some of the more controversial and tragic aspects of Haber's life. Shown here are the major figures in the cast in the 2005 Vancouver production of Vern Thiessen's play Einstein's Gift which centers on the conflict and tragedy in Fritz Haber's life. His first wife, Clara, was his former student and a chemist; Haber's decision to help Germany develop chlorine as a war gas led to her suicide.
See also Thomas Hager's 2008 book The alchemy of air; a Jewish genius, a doomed tycoon, and the scientific discovery that fed the world but fueled the rise of Hitler.
Many of the chemical reactions that occur in living organisms are regulated through the Le Châtelier principle.
Oxygen transport by the blood
Few of these are more important to warm-blooded organisms than those that relate to aerobic respiration, in which oxygen is transported to the cells where it is combined with glucose and metabolized to carbon dioxide, which then moves back to the lungs from which it is expelled.
hemoglobin + O2 ![]() oxyhemoglobin
oxyhemoglobin
The partial pressure of O2 in the air is 0.2 atm, sufficient to allow these molecules to be taken up by hemoglobin (the red pigment of blood) in which it becomes loosely bound in a complex known as oxyhemoglobin. At the ends of the capillaries which deliver the blood to the tissues, the O2 concentration is reduced by about 50% owing to its consumption by the cells. This shifts the equilibrium to the left, releasing the oxygen so it can diffuse into the cells.
Maintenance of blood pH
Carbon dioxide reacts with water to form a weak acid H2CO3 which would cause the blood pH to fall to dangerous levels if it were not promptly removed as it is excreted by the cells. This is accomplished by combining it with carbonate ion through the reaction
H2CO3 + CO32– ![]() 2 HCO3–
2 HCO3–
which is forced to the right by the high local CO2 concentration within the tissues. Once the hydrogen carbonate ("bicarbonate") ions reach the lung tissues where the CO2 partial pressure is much smaller, the reaction reverses and the CO2 is expelled.
Carbon monoxide poisoning
Carbon monoxide, a product of incomplete combustion that is present in automotive exhaust and cigarette smoke, binds to hemoglobin 200 times more tightly than does O2. This blocks the uptake and transport of oxygen by setting up a competing equilibrium
O2-hemoglobin ![]() hemoglobin
hemoglobin ![]() CO-hemoglobin
CO-hemoglobin
Air that contains as little as 0.1 percent carbon monoxide can tie up about half of the hemoglobin binding sites, reducing the amount of O2 reaching the tissues to fatal levels. Carbon monoxide poisoning is treated by administration of pure O2 which promotes the shift of the above equilibrium to the left. This can be made even more effective by placing the victim in a hyperbaric chamber in which the pressure of O2 can be made greater than 1 atm.
Make sure you thoroughly understand the following essential ideas which have been presented above. It is especially imortant that you know the precise meanings of all the highlighted terms in the context of this topic.
- A system in its equilibrium state will remain in that state indefinitely as long as it is undisturbed. If the equilibrium is destroyed by subjecting the system to a change of pressure, temperature, or the number of moles of a substance, then a net reaction will tend to take place that moves the system to a new equilibrium state. Le Châtelier's principle says that this net reaction will occur in a direction that partially offsets the change.
- The Le Châtelier Principle has practical effect only for reactions in which signficant quantities of both reactants and products are present at equilibrium— that is, for reactions that are thermodynamically reversible.
- Addition of more product substances to an equilibrium mixture will shift the equilibrium to the left; addition of more reactant substances will shift it to the right. These effects are easily explained in terms of competing forward- and reverse reactions— that is, by the law of mass action.
- If a reaction is exothermic (releases heat), an increase in the temperature will force the equilibrium to the left, causing the system to absorb heat and thus partially ofsetting the rise in temperature. The opposite effect occurs for endothermic reactions, which are shifted to the right by rising temperature.
- The effect of pressure on an equilibrium is significant only for reactions which involve different numbers of moles of gases on the two sides of the equation. If the number of moles of gases increases, than an increase in the total pressure will tend to initiate a reverse reaction that consumes some the products, partially reducing the effect of the pressure increase.
- The classic example of the practical use of the Le Châtelier principle is the Haber-Bosch process for the synthesis of ammonia, in which a balance between low temperature and high pressure must be found.
See here for another treatment of the Le Châtelier principle.
Henri Louis Le Châtelier: A man of principle - biographical sketch, including his original statement of the Principle.